The nature of protein folding pathways
- PMID: 25326421
- PMCID: PMC4234557
- DOI: 10.1073/pnas.1411798111
The nature of protein folding pathways
Abstract
How do proteins fold, and why do they fold in that way? This Perspective integrates earlier and more recent advances over the 50-y history of the protein folding problem, emphasizing unambiguously clear structural information. Experimental results show that, contrary to prior belief, proteins are multistate rather than two-state objects. They are composed of separately cooperative foldon building blocks that can be seen to repeatedly unfold and refold as units even under native conditions. Similarly, foldons are lost as units when proteins are destabilized to produce partially unfolded equilibrium molten globules. In kinetic folding, the inherently cooperative nature of foldons predisposes the thermally driven amino acid-level search to form an initial foldon and subsequent foldons in later assisted searches. The small size of foldon units, ∼ 20 residues, resolves the Levinthal time-scale search problem. These microscopic-level search processes can be identified with the disordered multitrack search envisioned in the "new view" model for protein folding. Emergent macroscopic foldon-foldon interactions then collectively provide the structural guidance and free energy bias for the ordered addition of foldons in a stepwise pathway that sequentially builds the native protein. These conclusions reconcile the seemingly opposed new view and defined pathway models; the two models account for different stages of the protein folding process. Additionally, these observations answer the "how" and the "why" questions. The protein folding pathway depends on the same foldon units and foldon-foldon interactions that construct the native structure.
Keywords: hydrogen exchange; protein folding; protein structure.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

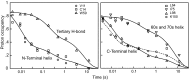

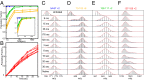
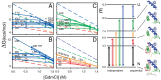

References
-
- Bai Y, Englander JJ, Mayne L, Milne JS, Englander SW. Thermodynamic parameters from hydrogen exchange measurements. Methods Enzymol. 1995;259:344–356. - PubMed
-
- Bai Y, Englander SW. Future directions in folding: The multi-state nature of protein structure. Proteins. 1996;24(2):145–151. - PubMed
-
- Levinthal C. Are there pathways for protein folding. J Chim Phys. 1968;65:44–45.
-
- Levinthal C. Mossbauer Spectroscopy in Biological Systems. Proceedings University of Illinois Bulletin. University of Illinois Press; Urbana, IL: 1969. How to fold graciously; pp. 22–24.
-
- Luheshi LM, Crowther DC, Dobson CM. Protein misfolding and disease: From the test tube to the organism. Curr Opin Chem Biol. 2008;12(1):25–31. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

