Novel mechanism coupling cyclic AMP-protein kinase A signaling and golgi trafficking via Gyp1 phosphorylation in polarized growth
- PMID: 25326521
- PMCID: PMC4248690
- DOI: 10.1128/EC.00231-14
Novel mechanism coupling cyclic AMP-protein kinase A signaling and golgi trafficking via Gyp1 phosphorylation in polarized growth
Abstract
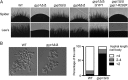
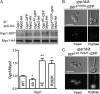
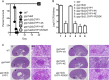
The cyclic AMP (cAMP)-protein kinase A (PKA) signaling activates virulence expression during hyphal development in the fungal human pathogen Candida albicans. The hyphal growth is characterized by Golgi polarization toward the hyphal tips, which is thought to enhance directional vesicle transport. However, how the hypha-induction signal regulates Golgi polarization is unknown. Gyp1, a Golgi-associated protein and the first GTPase-activating protein (GAP) in the Rab GAP cascade, critically regulates membrane trafficking from the endoplasmic reticulum to the plasma membrane. Here, we report a novel pathway by which the cAMP-PKA signaling triggers Golgi polarization during hyphal growth. We demonstrate that Gyp1 plays a crucial role in actin-dependent Golgi polarization. Hyphal induction activates PKA, which in turn phosphorylates Gyp1. Phosphomimetic mutation of four PKA sites identified by mass spectrometry (Gyp1(4E)) caused strong Gyp1 polarization to hyphal tips, whereas nonphosphorylatable mutations (Gyp1(4A)) abolished it. Gyp1(4E) exhibited enhanced association with the actin motor Myo2, while Gyp1(4A) showed the opposite effect, providing a possible mechanism for Golgi polarization. A GAP-dead Gyp1 (Gyp1(R292K)) showed strong polarization similar to that seen with Gyp1(4E), indicating a role for the GAP activity. Mutating the PKA sites on Gyp1 also impaired the recruitment of a late Golgi marker, Sec7. Furthermore, proper PKA phosphorylation and GAP activity of Gyp1 are required for virulence in mice. We propose that the cAMP-PKA signaling directly targets Gyp1 to promote Golgi polarization in the yeast-to-hypha transition, an event crucial for C. albicans infection.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures




Similar articles
-
Hgc1, a novel hypha-specific G1 cyclin-related protein regulates Candida albicans hyphal morphogenesis.EMBO J. 2004 Apr 21;23(8):1845-56. doi: 10.1038/sj.emboj.7600195. Epub 2004 Apr 8. EMBO J. 2004. PMID: 15071502 Free PMC article.
-
The Ras/cAMP/PKA signaling pathway and virulence in Candida albicans.Future Microbiol. 2009 Dec;4(10):1263-70. doi: 10.2217/fmb.09.106. Future Microbiol. 2009. PMID: 19995187 Review.
-
The G protein-coupled receptor Gpr1 and the Galpha protein Gpa2 act through the cAMP-protein kinase A pathway to induce morphogenesis in Candida albicans.Mol Biol Cell. 2005 Apr;16(4):1971-86. doi: 10.1091/mbc.e04-09-0780. Epub 2005 Jan 26. Mol Biol Cell. 2005. PMID: 15673611 Free PMC article.
-
The Set3/Hos2 histone deacetylase complex attenuates cAMP/PKA signaling to regulate morphogenesis and virulence of Candida albicans.PLoS Pathog. 2010 May 13;6(5):e1000889. doi: 10.1371/journal.ppat.1000889. PLoS Pathog. 2010. PMID: 20485517 Free PMC article.
-
To each its own: Mechanisms of cross-talk between GPI biosynthesis and cAMP-PKA signaling in Candida albicans versus Saccharomyces cerevisiae.J Biol Chem. 2024 Jul;300(7):107444. doi: 10.1016/j.jbc.2024.107444. Epub 2024 Jun 4. J Biol Chem. 2024. PMID: 38838772 Free PMC article. Review.
Cited by
-
Candida albicans Hyphae: From Growth Initiation to Invasion.J Fungi (Basel). 2018 Jan 11;4(1):10. doi: 10.3390/jof4010010. J Fungi (Basel). 2018. PMID: 29371503 Free PMC article. Review.
-
The GTPase-Activating Protein FgGyp1 Is Important for Vegetative Growth, Conidiation, and Virulence and Negatively Regulates DON Biosynthesis in Fusarium graminearium.Front Microbiol. 2021 Jan 21;12:621519. doi: 10.3389/fmicb.2021.621519. eCollection 2021. Front Microbiol. 2021. PMID: 33552040 Free PMC article.
-
New "haploid biofilm model" unravels IRA2 as a novel regulator of Candida albicans biofilm formation.Sci Rep. 2015 Jul 23;5:12433. doi: 10.1038/srep12433. Sci Rep. 2015. PMID: 26202015 Free PMC article.
-
Sac7 and Rho1 regulate the white-to-opaque switching in Candida albicans.Sci Rep. 2018 Jan 17;8(1):875. doi: 10.1038/s41598-018-19246-9. Sci Rep. 2018. PMID: 29343748 Free PMC article.
-
Hgc1-Cdc28-how much does a single protein kinase do in the regulation of hyphal development in Candida albicans?J Microbiol. 2016 Mar;54(3):170-7. doi: 10.1007/s12275-016-5550-9. Epub 2016 Feb 27. J Microbiol. 2016. PMID: 26920877 Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

