Silencing of high-mobility group box 2 (HMGB2) modulates cisplatin and 5-fluorouracil sensitivity in head and neck squamous cell carcinoma
- PMID: 25327479
- PMCID: PMC4528963
- DOI: 10.1002/pmic.201400338
Silencing of high-mobility group box 2 (HMGB2) modulates cisplatin and 5-fluorouracil sensitivity in head and neck squamous cell carcinoma
Abstract
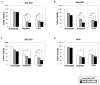
Dysregulation of protein expression is associated with most diseases including cancer. MS-based proteomic analysis is widely employed as a tool to study protein dysregulation in cancers. Proteins that are differentially expressed in head and neck squamous cell carcinoma (HNSCC) cell lines compared to the normal oral cell line could serve as biomarkers for patient stratification. To understand the proteomic complexity in HNSCC, we carried out iTRAQ-based MS analysis on a panel of HNSCC cell lines in addition to a normal oral keratinocyte cell line. LC-MS/MS analysis of total proteome of the HNSCC cell lines led to the identification of 3263 proteins, of which 185 proteins were overexpressed and 190 proteins were downregulated more than twofold in at least two of the three HNSCC cell lines studied. Among the overexpressed proteins, 23 proteins were related to DNA replication and repair. These included high-mobility group box 2 (HMGB2) protein, which was overexpressed in all three HNSCC lines studied. Overexpression of HMGB2 has been reported in various cancers, yet its role in HNSCC remains unclear. Immunohistochemical labeling of HMGB2 in a panel of HNSCC tumors using tissue microarrays revealed overexpression in 77% (54 of 70) of tumors. The HMGB proteins are known to bind to DNA structure resulting from cisplatin-DNA adducts and affect the chemosensitivity of cells. We observed that siRNA-mediated silencing of HMGB2 increased the sensitivity of the HNSCC cell lines to cisplatin and 5-FU. We hypothesize that targeting HMGB2 could enhance the efficacy of existing chemotherapeutic regimens for treatment of HNSCC. All MS data have been deposited in the ProteomeXchange with identifier PXD000737 (http://proteomecentral.proteomexchange.org/dataset/PXD000737).
Keywords: Antimetabolite; Biomarker; Biomedicine; Drug resistance; In vitro labeling.
© 2014 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
The authors have declared no conflict of interest.
Figures



References
-
- Leemans CR, Braakhuis BJ, Brakenhoff RH. The molecular biology of head and neck cancer. Nat Rev Cancer. 2011;11:9–22. - PubMed
-
- Yarbrough WG, Slebos RJ, Liebler D. Proteomics: clinical applications for head and neck squamous cell carcinoma. Head & neck. 2006;28:549–558. - PubMed
-
- Matta A, Ralhan R, DeSouza LV, Siu KW. Mass spectrometry-based clinical proteomics: head-and-neck cancer biomarkers and drug-targets discovery. Mass Spectrom Rev. 2010;29:945–961. - PubMed
-
- Marimuthu A, Chavan S, Sathe G, Sahasrabuddhe NA, et al. Identification of head and neck squamous cell carcinoma biomarker candidates through proteomic analysis of cancer cell secretome. Biochimica et biophysica acta. 2013;1834:2308–2316. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

