Prospective evaluation of the feasibility of sentinel lymph node biopsy in breast cancer patients with negative axillary conversion after neoadjuvant chemotherapy
- PMID: 25327493
- PMCID: PMC4296849
- DOI: 10.4143/crt.2013.208
Prospective evaluation of the feasibility of sentinel lymph node biopsy in breast cancer patients with negative axillary conversion after neoadjuvant chemotherapy
Abstract
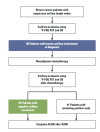
Purpose: Tumor response to neoadjuvant chemotherapy (NAC) may adversely affect the identification and accuracy rate of sentinel lymph node biopsy (SLNB). This study was conducted to evaluate the feasibility of SLNB in node-positive breast cancer patients with negative axillary conversion after NAC.
Materials and methods: Ninety-six patients with positive nodes at presentation were prospectively enrolled. (18)Fluorodeoxyglucose-positron emission tomography ((18)F-FDG PET) and ultrasonography were performed before and after NAC. A metastatic axillary lymph node was defined as positive if it was positive upon both (18)F-FDG PET and ultrasonography, while it was considered negative if it was negative upon both (18)F-FDG PET and ultrasonography.
Results: After NAC, 55 cases (57.3%) became clinically node-negative, while 41 cases (42.7%) remained node-positive. In the entire cohort, the sentinel lymph node (SLN) identification and false-negative rates were 84.3% (81/96) and 18.4% (9/49), respectively. In the negative axillary conversion group, the results of SLNB showed an 85.7% (48/55) identification rate and 16.7% (4/24) false-negative rate.
Conclusion: For breast cancer patients with clinically positive nodes at presentation, it is difficult to conclude whether the SLN accurately represents the metastatic status of all axillary lymph nodes, even after clinically negative node conversion following NAC.
Keywords: Breast neoplasms; Neoadjuvant therapy; Predictive value of tests; Sentinel lymph node biopsy.
Conflict of interest statement
Conflict of interest relevant to this article was not reported.
Figures

Similar articles
-
Update on sentinel lymph node biopsy after neoadjuvant chemotherapy in breast cancer patient.Ann Ital Chir. 2020;91:465-468. Ann Ital Chir. 2020. PMID: 32312945
-
Contrast-enhanced ultrasonography and blue dye methods in detection of sentinel lymph nodes following neoadjuvant chemotherapy in initially node positive breast cancer.Arch Gynecol Obstet. 2020 Sep;302(3):685-692. doi: 10.1007/s00404-020-05646-8. Epub 2020 Jun 29. Arch Gynecol Obstet. 2020. PMID: 32602000
-
Sentinel lymph node biopsy examination for breast cancer patients with clinically negative axillary lymph nodes after neoadjuvant chemotherapy.Am J Surg. 2006 Feb;191(2):225-9. doi: 10.1016/j.amjsurg.2005.06.049. Am J Surg. 2006. PMID: 16442950
-
Refining the Performance of Sentinel Lymph Node Biopsy Post-neoadjuvant Chemotherapy in Patients with Pathologically Proven Pre-treatment Node-positive Breast Cancer: An Update for Clinical Practice.Anticancer Res. 2016 Apr;36(4):1461-71. Anticancer Res. 2016. PMID: 27069121 Review.
-
The Feasibility and Reliability of Sentinel Lymph Node Biopsy After Neoadjuvant Chemotherapy in Breast Cancer Patients With Negative Axillary Lymph Nodes-A Meta-analysis.Breast Cancer (Auckl). 2024 May 30;18:11782234241255856. doi: 10.1177/11782234241255856. eCollection 2024. Breast Cancer (Auckl). 2024. PMID: 38826850 Free PMC article. Review.
Cited by
-
Performance of sentinel lymph node biopsy after neoadjuvant chemotherapy in clinically node-positive breast cancer patients: systematic review and meta-analysis.Int J Surg. 2025 Apr 1;111(4):3040-3050. doi: 10.1097/JS9.0000000000002275. Int J Surg. 2025. PMID: 39878175 Free PMC article.
-
Feasibility of Sentinel Lymph Node Biopsy in Breast Cancer Patients with Axillary Conversion after Neoadjuvant Chemotherapy-A Single-Tertiary Centre Experience and Review of the Literature.Diagnostics (Basel). 2023 Sep 20;13(18):3000. doi: 10.3390/diagnostics13183000. Diagnostics (Basel). 2023. PMID: 37761367 Free PMC article.
-
AGO Recommendations for the Surgical Therapy of the Axilla After Neoadjuvant Chemotherapy: 2021 Update.Geburtshilfe Frauenheilkd. 2021 Oct;81(10):1112-1120. doi: 10.1055/a-1499-8431. Epub 2021 Oct 6. Geburtshilfe Frauenheilkd. 2021. PMID: 34629490 Free PMC article.
-
A survey of current surgical treatment of early stage breast cancer in China.Oncoscience. 2018 Aug 22;5(7-8):239-247. doi: 10.18632/oncoscience.445. eCollection 2018 Jul. Oncoscience. 2018. PMID: 30234145 Free PMC article.
-
Axillary response according to neoadjuvant single or dual human epidermal growth factor receptor 2 (HER2) blockade in clinically node-positive, HER2-positive breast cancer.Int J Cancer. 2021 Oct 15;149(8):1585-1592. doi: 10.1002/ijc.33726. Epub 2021 Jul 8. Int J Cancer. 2021. PMID: 34213778 Free PMC article.
References
-
- Krag DN, Anderson SJ, Julian TB, Brown AM, Harlow SP, Ashikaga T, et al. Technical outcomes of sentinel-lymph-node resection and conventional axillary-lymph-node dissection in patients with clinically node-negative breast cancer: results from the NSABP B-32 randomised phase III trial. Lancet Oncol. 2007;8:881–8. - PubMed
-
- Lyman GH, Giuliano AE, Somerfield MR, Benson AB, 3rd, Bodurka DC, Burstein HJ, et al. American Society of Clinical Oncology guideline recommendations for sentinel lymph node biopsy in early-stage breast cancer. J Clin Oncol. 2005;23:7703–20. - PubMed
-
- Schwartz GF, Giuliano AE, Veronesi U, Consensus Conference Committee Proceedings of the consensus conference on the role of sentinel lymph node biopsy in carcinoma of the breast, April 19-22, 2001, Philadelphia, Pennsylvania. Cancer. 2002;94:2542–51. - PubMed
-
- Fisher B, Bryant J, Wolmark N, Mamounas E, Brown A, Fisher ER, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol. 1998;16:2672–85. - PubMed
LinkOut - more resources
Full Text Sources

