Formation of self-organized Zircaloy-4 oxide nanotubes in organic viscous electrolyte via anodization
- PMID: 25328503
- PMCID: PMC4199941
- DOI: 10.1186/1556-276X-9-553
Formation of self-organized Zircaloy-4 oxide nanotubes in organic viscous electrolyte via anodization
Abstract
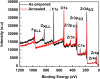
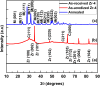
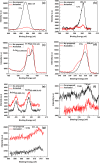
This work reports the formation of self-organized Zircaloy-4 (Zr-4) oxide nanotubes in viscous organic ethylene glycol (EG) electrolyte containing a small amount of fluoride salt and deionized (DI) water via an electrochemical anodization. The structure, morphology, and composition of the Zr-4 oxide nanotubes were studied using X-ray diffraction (XRD), scanning electron microscope (SEM), transmission electron microscope (TEM), EDX, and XPS. SEM results showed that the length of the nanotubes is approximately 13 μm, and TEM results showed that the inner diameter of the Zr-4 oxide nanotubes is approximately 20 nm with average wall thickness of approximately 7 nm. XRD and selected area electron diffraction pattern (SAED) results confirmed that the as-anodized Zr-4 oxide nanotubes have cubic crystalline structure. Both cubic and monoclinic phases were found after annealing of Zr-4 oxide nanotubes. The tubular structure morphology of Zr-4 oxide nanotubes did not remain intact after annealing which is attributed to the elimination of F species from the annealed nanotubes.
Keywords: Anodization; Nanotubes; SEM; TEM; XPS; Zircaloy.
Figures



References
-
- Billone M, Yan Y, Burtseva T, Daum R. Cladding Embrittlement during Postulated Loss of Coolant Accidents. NUREG/CR–6967 ANL–07/04. Argonne: Nuclear Engineering Division, Argonne National Laboratory;
-
- Chemelle P, Knorr DB, Sande Van Der JB, Pelloux RM. Morphology and composition of second phase particles in Zircaloy-2. J Nucl Mater. 1983;113:58.
-
- Ahmad M, Akhter JI, Ali G, Akhtar M, Choudhry MA. Characterization of electron beam modified surface of Zircaloy-4. J Alloys and Compd. 2006;426:176–179.
-
- Ahn HS, Lee C, Kim H, Jo HJ, Kang SH, Kim J, Shin J, Kim MH. Pool boiling CHF enhancement by micro/nanoscale modification of Zircaloy-4 surface. Nucl Eng Des. 2010;240:3350–3360.
-
- Lee C, Kim H, Ahn HS, Kim MH, Kim J. Micro/nanostructure evolution of Zircaloy surface using anodization technique: application to nuclear fuel cladding modification. App Sur Sci. 2012;258:8724–8731.
LinkOut - more resources
Full Text Sources
Other Literature Sources

