PROPHYLACTIC ANTIBODY TREATMENT AND INTRAMUSCULAR IMMUNIZATION REDUCE INFECTIOUS HUMAN RHINOVIRUS 16 LOAD IN THE LOWER RESPIRATORY TRACT OF CHALLENGED COTTON RATS
- PMID: 25328560
- PMCID: PMC4199241
- DOI: 10.1016/j.trivac.2014.02.003
PROPHYLACTIC ANTIBODY TREATMENT AND INTRAMUSCULAR IMMUNIZATION REDUCE INFECTIOUS HUMAN RHINOVIRUS 16 LOAD IN THE LOWER RESPIRATORY TRACT OF CHALLENGED COTTON RATS
Abstract
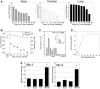
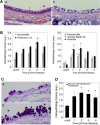
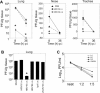
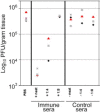
Human rhinoviruses (HRV) represent the single most important etiological agents of the common cold and are the most frequent cause of acute respiratory infections in humans. Currently the performance of available animal models for immunization studies using HRV challenge is very limited. The cotton rat (Sigmodon hispidus) is a well-recognized model for the study of human respiratory viral infections. In this work we show that, without requiring any genetic modification of either the host or the virus, intranasal infection of cotton rats with HRV16 resulted in measurable lower respiratory tract pathology, mucus production, and expression of interferon-activated genes. Intramuscular immunization with live HRV16 generated robust protective immunity that correlated with high serum levels of neutralizing antibodies. In addition, cotton rats treated prophylactically with hyperimmune anti-HRV16 serum were protected against HRV16 intranasal challenge. Finally, protection by immunization was efficiently transferred from mothers to newborn animals resulting in a substantial reduction of infectious virus loads in the lung following intranasal challenge. Overall, our results demonstrate that the cotton rat provides valuable additional model development options for testing vaccines and prophylactic therapies against rhinovirus infection.
Figures

References
-
- Gern J.E., Palmenberg A.C. Rhinoviruses. Chapter 18. In: Knipe D.M., Howley P.M., editors. sixth ed. vol. 1. Lippincott-Williams & Wilkins; Philadelphia: 2013. p. 531. (Fields Virology).
-
- Knowles N.J., Hovi T., Hyypiä T., King A.M.Q., Lindberg A.M. Picornaviridae. In: King A.M.Q., Adams M.J., Carstens E.B., Lefkowitz E.J., editors. Virus Taxonomy: Classification and Nomenclature of Viruses: Ninth Report of the International Committee on Taxonomy of Viruses. Elsevier; San Diego: 2012. pp. 855–880.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
