Neuregulin-1β induces mature ventricular cardiac differentiation from induced pluripotent stem cells contributing to cardiac tissue repair
- PMID: 25329043
- PMCID: PMC4313422
- DOI: 10.1089/scd.2014.0211
Neuregulin-1β induces mature ventricular cardiac differentiation from induced pluripotent stem cells contributing to cardiac tissue repair
Abstract
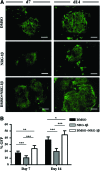
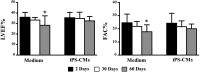
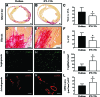
Stem cell-derived cardiomyocytes (CMs) are often electrophysiologically immature and heterogeneous, which represents a major barrier to their in vitro and in vivo application. Therefore, the purpose of this study was to examine whether Neuregulin-1β (NRG-1β) treatment could enhance in vitro generation of mature "working-type" CMs from induced pluripotent stem (iPS) cells and assess the regenerative effects of these CMs on cardiac tissue after acute myocardial infarction (AMI). With that purpose, adult mouse fibroblast-derived iPS from α-MHC-GFP mice were derived and differentiated into CMs through NRG-1β and/or dimethyl sulfoxide (DMSO) treatment. Cardiac specification and maturation of the iPS was analyzed by gene expression array, quantitative real-time polymerase chain reaction, immunofluorescence, electron microscopy, and patch-clamp techniques. In vivo, the iPS-derived CMs or culture medium control were injected into the peri-infarct region of hearts after coronary artery ligation, and functional and histology changes were assessed from 1 to 8 weeks post-transplantation. On differentiation, the iPS displayed early and robust in vitro cardiogenesis, expressing cardiac-specific genes and proteins. More importantly, electrophysiological studies demonstrated that a more mature ventricular-like cardiac phenotype was achieved when cells were treated with NRG-1β and DMSO compared with DMSO alone. Furthermore, in vivo studies demonstrated that iPS-derived CMs were able to engraft and electromechanically couple to heart tissue, ultimately preserving cardiac function and inducing adequate heart tissue remodeling. In conclusion, we have demonstrated that combined treatment with NRG-1β and DMSO leads to efficient differentiation of iPS into ventricular-like cardiac cells with a higher degree of maturation, which are capable of preserving cardiac function and tissue viability when transplanted into a mouse model of AMI.
Figures


Similar articles
-
Generation of electrophysiologically functional cardiomyocytes from mouse induced pluripotent stem cells.Stem Cell Res. 2016 Mar;16(2):522-30. doi: 10.1016/j.scr.2016.02.032. Epub 2016 Feb 23. Stem Cell Res. 2016. PMID: 26972055 Free PMC article.
-
Efficient Cardiac Differentiation of Human Amniotic Fluid-Derived Stem Cells into Induced Pluripotent Stem Cells and Their Potential Immune Privilege.Int J Mol Sci. 2020 Mar 29;21(7):2359. doi: 10.3390/ijms21072359. Int J Mol Sci. 2020. PMID: 32235313 Free PMC article.
-
Susceptibility of murine induced pluripotent stem cell-derived cardiomyocytes to hypoxia and nutrient deprivation.Stem Cell Res Ther. 2015 Apr 23;6(1):83. doi: 10.1186/s13287-015-0057-6. Stem Cell Res Ther. 2015. PMID: 25900017 Free PMC article.
-
Induced pluripotent stem cells as a new strategy for cardiac regeneration and disease modeling.J Mol Cell Cardiol. 2013 Sep;62:43-50. doi: 10.1016/j.yjmcc.2013.04.022. Epub 2013 Apr 30. J Mol Cell Cardiol. 2013. PMID: 23643470 Review.
-
iPS cells: a source of cardiac regeneration.J Mol Cell Cardiol. 2011 Feb;50(2):327-32. doi: 10.1016/j.yjmcc.2010.10.026. Epub 2010 Oct 30. J Mol Cell Cardiol. 2011. PMID: 21040726 Review.
Cited by
-
Tissue engineering the cardiac microenvironment: Multicellular microphysiological systems for drug screening.Adv Drug Deliv Rev. 2016 Jan 15;96:225-33. doi: 10.1016/j.addr.2015.07.004. Epub 2015 Jul 23. Adv Drug Deliv Rev. 2016. PMID: 26212156 Free PMC article. Review.
-
Overexpression of KCNJ2 enhances maturation of human-induced pluripotent stem cell-derived cardiomyocytes.Stem Cell Res Ther. 2023 Apr 15;14(1):92. doi: 10.1186/s13287-023-03312-9. Stem Cell Res Ther. 2023. PMID: 37061738 Free PMC article.
-
Novel Biological Therapies Targeting Heart Failure: Myocardial Rejuvenation.Heart Fail Clin. 2016 Jul;12(3):461-71. doi: 10.1016/j.hfc.2016.03.008. Epub 2016 May 10. Heart Fail Clin. 2016. PMID: 27371521 Free PMC article. Review.
-
Maturation strategies and limitations of induced pluripotent stem cell-derived cardiomyocytes.Biosci Rep. 2021 Jun 25;41(6):BSR20200833. doi: 10.1042/BSR20200833. Biosci Rep. 2021. PMID: 33057659 Free PMC article. Review.
-
Micromanaging cardiac regeneration: Targeted delivery of microRNAs for cardiac repair and regeneration.World J Cardiol. 2016 Feb 26;8(2):163-79. doi: 10.4330/wjc.v8.i2.163. World J Cardiol. 2016. PMID: 26981212 Free PMC article. Review.
References
-
- Passier R, van Laake LW. and Mummery CL. (2008). Stem-cell-based therapy and lessons from the heart. Nature 453:322–329 - PubMed
-
- Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, et al. (2003). Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell 114:763–776 - PubMed
-
- Winter EM, van Oorschot AA, Hogers B, van der Graaf LM, Doevendans PA, Poelmann RE, Atsma DE, Gittenberger-de Groot AC. and Goumans MJ. (2009). A new direction for cardiac regeneration therapy: application of synergistically acting epicardium-derived cells and cardiomyocyte progenitor cells. Circ Heart Fail 2:643–653 - PubMed
-
- van Laake LW, Passier R, Monshouwer-Kloots J, Verkleij AJ, Lips DJ, Freund C, den Ouden K, Ward-van Oostwaard D, Korving J, et al. (2007). Human embryonic stem cell-derived cardiomyocytes survive and mature in the mouse heart and transiently improve function after myocardial infarction. Stem Cell Res 1:9–24 - PubMed
-
- Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, Reinecke H, Xu C, Hassanipour M, et al. (2007). Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol 25:1015–1024 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

