Protective efficacy of a Plasmodium vivax circumsporozoite protein-based vaccine in Aotus nancymaae is associated with antibodies to the repeat region
- PMID: 25329054
- PMCID: PMC4199514
- DOI: 10.1371/journal.pntd.0003268
Protective efficacy of a Plasmodium vivax circumsporozoite protein-based vaccine in Aotus nancymaae is associated with antibodies to the repeat region
Abstract
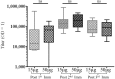
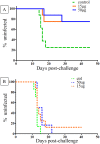
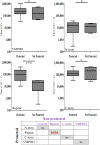
We have previously reported that Vivax Malaria Protein 001 (VMP001), a vaccine candidate based on the circumsporozoite protein of Plasmodium vivax, is immunogenic in mice and rhesus monkeys in the presence of various adjuvants. In the present study, we evaluated the immunogenicity and efficacy of VMP001 formulated with a TLR9 agonist in a water-in-oil emulsion. Following immunization, the vaccine efficacy was assessed by challenging Aotus nancymaae monkeys with P. vivax sporozoites. Monkeys from both the low- and high-dose vaccine groups generated strong humoral immune responses to the vaccine (peak median titers of 291,622), and its subunits (peak median titers to the N-term, central repeat and C-term regions of 22,188; 66,120 and 179,947, respectively). 66.7% of vaccinated monkeys demonstrated sterile protection following challenge. Protection was associated with antibodies directed against the central repeat region. The protected monkeys had a median anti-repeat titer of 97,841 compared to 14,822 in the non-protected monkeys. This is the first report demonstrating P. vivax CSP vaccine-induced protection of Aotus monkeys challenged with P. vivax sporozoites.
Conflict of interest statement
On behalf of the government of The United States of America, as represented by the Secretary of the Army, AY and CFO are listed as inventors on issued and accepted patents on VMP001. These did not influence the study design, conduct, or data analysis. None of the other authors declare any conflict. This does not alter our adherence to all PLOS policies on sharing data and materials.
Figures



References
-
- Howes RE, Battle KE, Satyagraha AW, Baird JK, Hay SI (2013) G6PD deficiency: global distribution, genetic variants and primaquine therapy. Adv Parasitol 81: 133–201. - PubMed
-
- Bennett JW, Pybus BS, Yadava A, Tosh D, Sousa JC, et al. (2013) Primaquine failure and cytochrome P-450 2D6 in Plasmodium vivax malaria. N Engl J Med 369: 1381–1382. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

