Macrophage migration inhibitory factor is involved in ectopic endometrial tissue growth and peritoneal-endometrial tissue interaction in vivo: a plausible link to endometriosis development
- PMID: 25329068
- PMCID: PMC4201552
- DOI: 10.1371/journal.pone.0110434
Macrophage migration inhibitory factor is involved in ectopic endometrial tissue growth and peritoneal-endometrial tissue interaction in vivo: a plausible link to endometriosis development
Abstract
Pelvic inflammation is a hallmark of endometriosis pathogenesis and a major cause of the disease's symptoms. Abnormal immune and inflammatory changes may not only contribute to endometriosis-major symptoms, but also contribute to ectopic endometrial tissue growth and endometriosis development. A major pro-inflammatory factors found elevated in peritoneal fluid of women with endometriosis and to be overexpressed in peritoneal fluid macrophages and active, highly vascularized and early stage endometriotic lesions, macrophage migration inhibitory factor (MIF) appeared to induce angiogenic and inflammatory and estrogen producing phenotypes in endometriotic cells in vitro and to be a possible therapeutic target in vivo. Using a mouse model where MIF-knock out (KO) mice received intra-peritoneal injection of endometrial tissue from MIF-KO or syngeneic wild type (WT) mice and vice versa, our current study revealed that MIF genetic depletion resulted in a marked reduction ectopic endometrial tissue growth, a disrupted tissue structure and a significant down regulation of the expression of major inflammatory (cyclooxygenease-2), cell adhesion (αv and β3 integrins), survival (B-cell lymphoma-2) and angiogenic (vascular endothelial cell growth) factors relevant to endometriosis pathogenesis, whereas MIF add-back to MIF-KO mice significantly restored endometriosis-like lesions number and size. Interestingly, cross-experiments revealed that MIF presence in both endometrial and peritoneal host tissues is required for ectopic endometrial tissue growth and pointed to its involvement in endometrial-peritoneal interactions. This study provides compelling evidence for the role of MIF in endometriosis development and its possible interest for a targeted treatment of endometriosis.
Conflict of interest statement
Figures
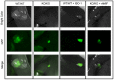
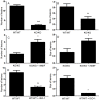
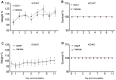


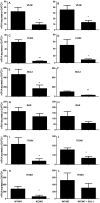

References
-
- Sampson J (1927) Peritoneal endometriosis due to menstrual dissemination of endometrial tisue into the peritoneal cavity. Am J Obstet Gynecol 14: 422–469.
-
- Berbic M, Fraser IS (2013) Immunology of normal and abnormal menstruation. Womens Health (Lond Engl) 9: 387–395. - PubMed
-
- Khoufache K, Michaud N, Harir N, Kibangou Bondza P, Akoum A (2012) Anomalies in the inflammatory response in endometriosis and possible consequences: a review. Minerva Endocrinol 37: 75–92. - PubMed
-
- Veillat V, Sengers V, Metz CN, Roger T, Leboeuf M, et al. (2012) Macrophage migration inhibitory factor is involved in a positive feedback loop increasing aromatase expression in endometriosis. Am J Pathol 181: 917–927. - PubMed
-
- Leng L, Bucala R (2006) Insight into the biology of macrophage migration inhibitory factor (MIF) revealed by the cloning of its cell surface receptor. Cell Res 16: 162–168. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

