Chemokine mediated monocyte trafficking into the retina: role of inflammation in alteration of the blood-retinal barrier in diabetic retinopathy
- PMID: 25329075
- PMCID: PMC4203688
- DOI: 10.1371/journal.pone.0108508
Chemokine mediated monocyte trafficking into the retina: role of inflammation in alteration of the blood-retinal barrier in diabetic retinopathy
Abstract
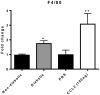
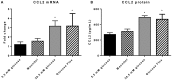
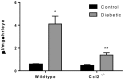
Inflammation in the diabetic retina is mediated by leukocyte adhesion to the retinal vasculature and alteration of the blood-retinal barrier (BRB). We investigated the role of chemokines in the alteration of the BRB in diabetes. Animals were made diabetic by streptozotocin injection and analyzed for gene expression and monocyte/macrophage infiltration. The expression of CCL2 (chemokine ligand 2) was significantly up-regulated in the retinas of rats with 4 and 8 weeks of diabetes and also in human retinal endothelial cells treated with high glucose and glucose flux. Additionally, diabetes or intraocular injection of recombinant CCL2 resulted in increased expression of the macrophage marker, F4/80. Cell culture impedance sensing studies showed that purified CCL2 was unable to alter the integrity of the human retinal endothelial cell barrier, whereas monocyte conditioned medium resulted in significant reduction in cell resistance, suggesting the relevance of CCL2 in early immune cell recruitment for subsequent barrier alterations. Further, using Cx3cr1-GFP mice, we found that intraocular injection of CCL2 increased retinal GFP+ monocyte/macrophage infiltration. When these mice were made diabetic, increased infiltration of monocytes/macrophages was also present in retinal tissues. Diabetes and CCL2 injection also induced activation of retinal microglia in these animals. Quantification by flow cytometry demonstrated a two-fold increase of CX3CR1+/CD11b+ (monocyte/macrophage and microglia) cells in retinas of wildtype diabetic animals in comparison to control non-diabetic ones. Using CCL2 knockout (Ccl2-/-) mice, we show a significant reduction in retinal vascular leakage and monocyte infiltration following induction of diabetes indicating the importance of this chemokine in alteration of the BRB. Thus, CCL2 may be an important therapeutic target for the treatment of diabetic macular edema.
Conflict of interest statement
Figures



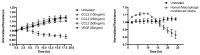

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

