Stabilization of homoserine-O-succinyltransferase (MetA) decreases the frequency of persisters in Escherichia coli under stressful conditions
- PMID: 25329174
- PMCID: PMC4201533
- DOI: 10.1371/journal.pone.0110504
Stabilization of homoserine-O-succinyltransferase (MetA) decreases the frequency of persisters in Escherichia coli under stressful conditions
Abstract
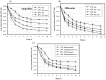
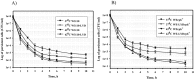
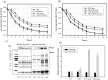
Bacterial persisters are a small subpopulation of cells that exhibit multi-drug tolerance without genetic changes. Generally, persistence is associated with a dormant state in which the microbial cells are metabolically inactive. The bacterial response to unfavorable environmental conditions (heat, oxidative, acidic stress) induces the accumulation of aggregated proteins and enhances formation of persister cells in Escherichia coli cultures. We have found that methionine supplementation reduced the frequency of persisters at mild (37°C) and elevated (42°C) temperatures, as well as in the presence of acetate. Homoserine-o-succinyltransferase (MetA), the first enzyme in the methionine biosynthetic pathway, is prone to aggregation under many stress conditions, resulting in a methionine limitation in E. coli growth. Overexpression of MetA induced the greatest number of persisters at 42°C, which is correlated to an increased level of aggregated MetA. Substitution of the native metA gene on the E. coli K-12 WE chromosome by a mutant gene encoding the stabilized MetA led to reduction in persisters at the elevated temperature and in the presence of acetate, as well as lower aggregation of the mutated MetA. Decreased persister formation at 42°C was confirmed also in E. coli K-12 W3110 and a fast-growing WErph+ mutant harboring the stabilized MetA. Thus, this is the first study to demonstrate manipulation of persister frequency under stressful conditions by stabilization of a single aggregation-prone protein, MetA.
Conflict of interest statement
Figures



Similar articles
-
Stabilized homoserine o-succinyltransferases (MetA) or L-methionine partially recovers the growth defect in Escherichia coli lacking ATP-dependent proteases or the DnaK chaperone.BMC Microbiol. 2013 Jul 30;13:179. doi: 10.1186/1471-2180-13-179. BMC Microbiol. 2013. PMID: 23898868 Free PMC article.
-
Improved thermostability and acetic acid tolerance of Escherichia coli via directed evolution of homoserine o-succinyltransferase.Appl Environ Microbiol. 2008 Dec;74(24):7660-8. doi: 10.1128/AEM.00654-08. Epub 2008 Oct 31. Appl Environ Microbiol. 2008. PMID: 18978085 Free PMC article.
-
Regulation of homoserine O-succinyltransferase for efficient production of L-methionine in engineered Escherichia coli.J Biotechnol. 2020 Feb 10;309:53-58. doi: 10.1016/j.jbiotec.2019.12.018. Epub 2019 Dec 28. J Biotechnol. 2020. PMID: 31891734
-
[Advances in the biosynthesis of L-homoserine and its derivatives by metabolic engineering of Escherichia coli].Sheng Wu Gong Cheng Xue Bao. 2022 Dec 25;38(12):4385-4402. doi: 10.13345/j.cjb.220377. Sheng Wu Gong Cheng Xue Bao. 2022. PMID: 36593184 Review. Chinese.
-
Multidrug tolerance of biofilms and persister cells.Curr Top Microbiol Immunol. 2008;322:107-31. doi: 10.1007/978-3-540-75418-3_6. Curr Top Microbiol Immunol. 2008. PMID: 18453274 Review.
Cited by
-
Stationary phase persister formation in Escherichia coli can be suppressed by piperacillin and PBP3 inhibition.BMC Microbiol. 2019 Jun 24;19(1):140. doi: 10.1186/s12866-019-1506-7. BMC Microbiol. 2019. PMID: 31234796 Free PMC article.
-
Regulation of sulfur starvation-induced proteins.3 Biotech. 2025 May;15(5):130. doi: 10.1007/s13205-025-04303-8. Epub 2025 Apr 16. 3 Biotech. 2025. PMID: 40255445 Review.
-
MetA is a "thermal fuse" that inhibits growth and protects Escherichia coli at elevated temperatures.Cell Rep. 2022 Aug 30;40(9):111290. doi: 10.1016/j.celrep.2022.111290. Cell Rep. 2022. PMID: 36044860 Free PMC article.
-
Protein Aggregation as a Bacterial Strategy to Survive Antibiotic Treatment.Front Mol Biosci. 2021 Apr 16;8:669664. doi: 10.3389/fmolb.2021.669664. eCollection 2021. Front Mol Biosci. 2021. PMID: 33937340 Free PMC article. Review.
-
A Gene Cluster That Encodes Histone Deacetylase Inhibitors Contributes to Bacterial Persistence and Antibiotic Tolerance in Burkholderia thailandensis.mSystems. 2020 Feb 11;5(1):e00609-19. doi: 10.1128/mSystems.00609-19. mSystems. 2020. PMID: 32047060 Free PMC article.
References
-
- Bigger JW (1944) Treatment of staphylococcal infections with penicillin by intermittent sterilisation. The Lancet 244: 497–500.
-
- Lewis K (2010) Persister cells. Annu Rev Microbiol 64: 357–372. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

