The contamination of commercial 15N2 gas stocks with 15N-labeled nitrate and ammonium and consequences for nitrogen fixation measurements
- PMID: 25329300
- PMCID: PMC4201487
- DOI: 10.1371/journal.pone.0110335
The contamination of commercial 15N2 gas stocks with 15N-labeled nitrate and ammonium and consequences for nitrogen fixation measurements
Abstract
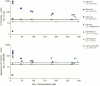
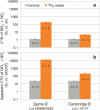
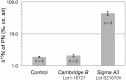
We report on the contamination of commercial 15-nitrogen (15N) N2 gas stocks with 15N-enriched ammonium, nitrate and/or nitrite, and nitrous oxide. 15N2 gas is used to estimate N2 fixation rates from incubations of environmental samples by monitoring the incorporation of isotopically labeled 15N2 into organic matter. However, the microbial assimilation of bioavailable 15N-labeled N2 gas contaminants, nitrate, nitrite, and ammonium, is liable to lead to the inflation or false detection of N2 fixation rates. 15N2 gas procured from three major suppliers was analyzed for the presence of these 15N-contaminants. Substantial concentrations of 15N-contaminants were detected in four Sigma-Aldrich 15N2 lecture bottles from two discrete batch syntheses. Per mole of 15N2 gas, 34 to 1900 µmoles of 15N-ammonium, 1.8 to 420 µmoles of 15N-nitrate/nitrite, and ≥21 µmoles of 15N-nitrous oxide were detected. One 15N2 lecture bottle from Campro Scientific contained ≥11 µmoles of 15N-nitrous oxide per mole of 15N2 gas, and no detected 15N-nitrate/nitrite at the given experimental 15N2 tracer dilutions. Two Cambridge Isotopes lecture bottles from discrete batch syntheses contained ≥0.81 µmoles 15N-nitrous oxide per mole 15N2, and trace concentrations of 15N-ammonium and 15N-nitrate/nitrite. 15N2 gas equilibrated cultures of the green algae Dunaliella tertiolecta confirmed that the 15N-contaminants are assimilable. A finite-differencing model parameterized using oceanic field conditions typical of N2 fixation assays suggests that the degree of detected 15N-ammonium contamination could yield inferred N2 fixation rates ranging from undetectable, <0.01 nmoles N L(-1) d(-1), to 530 nmoles N L(-1) d(-1), contingent on experimental conditions. These rates are comparable to, or greater than, N2 fixation rates commonly detected in field assays. These results indicate that past reports of N2 fixation should be interpreted with caution, and demonstrate that the purity of commercial 15N2 gas must be ensured prior to use in future N2 fixation rate determinations.
Conflict of interest statement
Figures

References
-
- Burris RH, Miller CE (1941) Application of N15 to the study of biological nitrogen fixation. Science 93(2405): 114–5. - PubMed
-
- Hardy RWF, Burns RC, Holsten RD (1973) Applications of the acetylene-ethylene assay for measurement of nitrogen fixation. Soil Biol Biochem 5(1): 47–81.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

