A sialic acid binding site in a human picornavirus
- PMID: 25329320
- PMCID: PMC4199766
- DOI: 10.1371/journal.ppat.1004401
A sialic acid binding site in a human picornavirus
Abstract
The picornaviruses coxsackievirus A24 variant (CVA24v) and enterovirus 70 (EV70) cause continued outbreaks and pandemics of acute hemorrhagic conjunctivitis (AHC), a highly contagious eye disease against which neither vaccines nor antiviral drugs are currently available. Moreover, these viruses can cause symptoms in the cornea, upper respiratory tract, and neurological impairments such as acute flaccid paralysis. EV70 and CVA24v are both known to use 5-N-acetylneuraminic acid (Neu5Ac) for cell attachment, thus providing a putative link between the glycan receptor specificity and cell tropism and disease. We report the structures of an intact human picornavirus in complex with a range of glycans terminating in Neu5Ac. We determined the structure of the CVA24v to 1.40 Å resolution, screened different glycans bearing Neu5Ac for CVA24v binding, and structurally characterized interactions with candidate glycan receptors. Biochemical studies verified the relevance of the binding site and demonstrated a preference of CVA24v for α2,6-linked glycans. This preference can be rationalized by molecular dynamics simulations that show that α2,6-linked glycans can establish more contacts with the viral capsid. Our results form an excellent platform for the design of antiviral compounds to prevent AHC.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
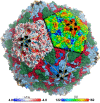
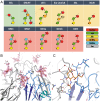
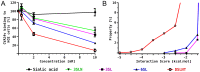
References
-
- Wright PW, Strauss GH, Langford MP (1992) Acute hemorrhagic conjunctivitis. Am Fam Physician 45: 173–178. - PubMed
-
- Aubry C, Gautret P, Nougairede A, Dussouil AS, Botelho-Nevers E, et al.. (2012) 2012 outbreak of acute haemorrhagic conjunctivitis in Indian Ocean Islands: identification of Coxsackievirus A24 in a returned traveller. Euro Surveill 17: : pii = 20185. - PubMed
-
- Cabrerizo M, Echevarria JE, Otero A, Lucas P, Trallero G (2008) Molecular characterization of a coxsackievirus A24 variant that caused an outbreak of acute haemorrhagic conjunctivitis in Spain, 2004. J Clin Virol 43: 323–327. - PubMed
-
- Ghazali O, Chua KB, Ng KP, Hooi PS, Pallansch MA, et al. (2003) An outbreak of acute haemorrhagic conjunctivitis in Melaka, Malaysia. Singapore Med J 44: 511–516. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

