Hepatotoxicity in mice of a novel anti-parasite drug candidate hydroxymethylnitrofurazone: a comparison with Benznidazole
- PMID: 25329323
- PMCID: PMC4199569
- DOI: 10.1371/journal.pntd.0003231
Hepatotoxicity in mice of a novel anti-parasite drug candidate hydroxymethylnitrofurazone: a comparison with Benznidazole
Abstract
Background: Treatment of Chagas disease, caused by Trypanosoma cruzi, relies on nifurtimox and benznidazole (BZL), which present side effects in adult patients, and natural resistance in some parasite strains. Hydroxymethylnitrofurazone (NFOH) is a new drug candidate with demonstrated trypanocidal activity; however, its safety is not known.
Methods: HepG2 cells dose response to NFOH and BZL (5-100 µM) was assessed by measurement of ROS, DNA damage and survival. Swiss mice were treated with NFOH or BZL for short-term (ST, 21 d) or long-term (LT, 60 d) periods. Sera levels of cellular injury markers, liver inflammatory and oxidative stress, and fibrotic remodeling were monitored.
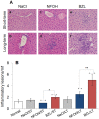
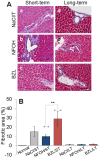
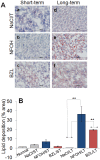
Results: HepG2 cells exhibited mild stress, evidenced by increased ROS and DNA damage, in response to NFOH, while BZL at 100 µM concentration induced >33% cell death in 24 h. In mice, NFOH ST treatment resulted in mild-to-no increase in the liver injury biomarkers (GOT, GPT), and liver levels of inflammatory (myeloperoxidase, TNF-α), oxidative (lipid peroxides) and nitrosative (3-nitrotyrosine) stress. These stress responses in NFOH LT treated mice were normalized to control levels. BZL-treated mice exhibited a >5-fold increase in GOT, GPT and TNF-α (LT) and a 20-40% increase in liver levels of MPO activity (ST and LT) in comparison with NFOH-treated mice. The liver inflammatory infiltrate was noted in the order of BZL>vehicle≥NFOH and BZL>NFOH≥vehicle, respectively, after ST and LT treatments. Liver fibrotic remodeling, identified after ST treatment, was in the order of BZL>vehicle>NFOH; lipid deposits, indicative of mitochondrial dysfunction and in the order of NFOH>vehicle>BZL were evidenced after LT treatment.
Conclusions: NFOH induces mild ST hepatotoxicity that is normalized during LT treatment in mice. Our results suggest that additional studies to determine the efficacy and toxicity of NFOH are warranted.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
Hydroxymethylnitrofurazone treatment in indeterminate form of chronic Chagas disease: Reduced intensity of tissue parasitism and inflammation-A histopathological study.Int J Exp Pathol. 2018 Oct;99(5):236-248. doi: 10.1111/iep.12289. Epub 2018 Oct 15. Int J Exp Pathol. 2018. PMID: 30320480 Free PMC article.
-
Hydroxymethylnitrofurazone is active in a murine model of Chagas' disease.Antimicrob Agents Chemother. 2010 Sep;54(9):3584-9. doi: 10.1128/AAC.01451-09. Epub 2010 Jun 21. Antimicrob Agents Chemother. 2010. PMID: 20566772 Free PMC article.
-
Low-dose benznidazole treatment results in parasite clearance and attenuates heart inflammatory reaction in an experimental model of infection with a highly virulent Trypanosoma cruzi strain.Int J Parasitol Drugs Drug Resist. 2015 Dec 12;6(1):12-22. doi: 10.1016/j.ijpddr.2015.12.001. eCollection 2016 Apr. Int J Parasitol Drugs Drug Resist. 2015. PMID: 26862474 Free PMC article.
-
The effect of benznidazole dose among the efficacy outcome in the murine animal model. A quantitative integration of the literature.Acta Trop. 2020 Jan;201:105218. doi: 10.1016/j.actatropica.2019.105218. Epub 2019 Oct 11. Acta Trop. 2020. PMID: 31610148 Review.
-
Treatment of Trypanosoma cruzi infection in the undetermined phase. Experience and current guidelines of treatment in Argentina.Mem Inst Oswaldo Cruz. 1999;94 Suppl 1:363-5. doi: 10.1590/s0074-02761999000700070. Mem Inst Oswaldo Cruz. 1999. PMID: 10677756 Review. No abstract available.
Cited by
-
Clomipramine and Benznidazole Act Synergistically and Ameliorate the Outcome of Experimental Chagas Disease.Antimicrob Agents Chemother. 2016 May 23;60(6):3700-8. doi: 10.1128/AAC.00404-16. Print 2016 Jun. Antimicrob Agents Chemother. 2016. PMID: 27067322 Free PMC article.
-
Hydroxymethylnitrofurazone treatment in indeterminate form of chronic Chagas disease: Reduced intensity of tissue parasitism and inflammation-A histopathological study.Int J Exp Pathol. 2018 Oct;99(5):236-248. doi: 10.1111/iep.12289. Epub 2018 Oct 15. Int J Exp Pathol. 2018. PMID: 30320480 Free PMC article.
-
ToxACoL: an endpoint-aware and task-focused compound representation learning paradigm for acute toxicity assessment.Nat Commun. 2025 Jul 1;16(1):5992. doi: 10.1038/s41467-025-60989-7. Nat Commun. 2025. PMID: 40593807 Free PMC article.
-
Albendazole induces oxidative stress and DNA damage in the parasitic protozoan Giardia duodenalis.Front Microbiol. 2015 Aug 6;6:800. doi: 10.3389/fmicb.2015.00800. eCollection 2015. Front Microbiol. 2015. PMID: 26300866 Free PMC article.
-
The trypanocidal benznidazole promotes adaptive response to oxidative injury: Involvement of the nuclear factor-erythroid 2-related factor-2 (Nrf2) and multidrug resistance associated protein 2 (MRP2).Toxicol Appl Pharmacol. 2016 Aug 1;304:90-8. doi: 10.1016/j.taap.2016.05.007. Epub 2016 May 12. Toxicol Appl Pharmacol. 2016. PMID: 27180241 Free PMC article.
References
-
- Schofield CJ, Jannin J, Salvatella R (2006) The future of Chagas disease control. Trends Parasitol 22: 583–588. - PubMed
-
- World Health Organization (2010) Chagas disease: control and elimination. UNDP/World Bank/WHO. http://apps.who.int/gb/ebwha/pdf_files/WHA63/A63_17-en.pdf, accessed 08/28/21014
-
- Maya JD, Orellana M, Ferreira J, Kemmerling U, Lopez-Munoz R, et al. (2010) Chagas disease: Present status of pathogenic mechanisms and chemotherapy. Biol Res 43: 323–331. - PubMed
-
- Urbina JA (2010) Specific chemotherapy of Chagas disease: relevance, current limitations and new approaches. Acta Trop 115: 55–68. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

