Bestrophin 3 ameliorates TNFα-induced inflammation by inhibiting NF-κB activation in endothelial cells
- PMID: 25329324
- PMCID: PMC4203846
- DOI: 10.1371/journal.pone.0111093
Bestrophin 3 ameliorates TNFα-induced inflammation by inhibiting NF-κB activation in endothelial cells
Abstract
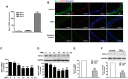
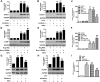
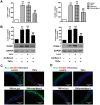
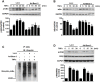
Increasing evidences have suggested vascular endothelial inflammatory processes are the initiator of atherosclerosis. Bestrophin 3 (Best-3) is involved in the regulation of cell proliferation, apoptosis and differentiation of a variety of physiological functions, but its function in cardiovascular system remains unclear. In this study, we investigated the effect of Best-3 on endothelial inflammation. We first demonstrated that Best-3 is expressed in endothelial cells and decreased after tumor necrosis factor-α (TNFα) challenge. Overexpression of Best-3 significantly attenuated TNFα-induced expression of adhesion molecules and chemokines, and subsequently inhibited the adhesion of monocytes to human umbilical vein endothelial cells (HUVECs). Conversely, knockdown of Best-3 with siRNA resulted in an enhancement on TNFα-induced expression of adhesion molecules and chemokines and adhesion of monocytes to HUVECs. Furthermore, overexpression of Best-3 with adenovirus dramatically ameliorated inflammatory response in TNFα-injected mice. Mechanistically, we found up-regulation of Best-3 inhibited TNFα-induced IKKβ and IκBα phosphorylation, IκBα degradation and NF-κB translocation. Our results demonstrated that Best-3 is an endogenous inhibitor of NF-κB signaling pathway in endothelial cells, suggesting that forced Best-3 expression may be a novel approach for the treatment of vascular inflammatory diseases.
Conflict of interest statement
Figures

References
-
- Ross R (1999) Atherosclerosis is an inflammatory disease. Am Heart J 138: S419–420. - PubMed
-
- Schiffrin EL (2002) Canadian Institutes of Health Research Multidisciplinary Research Group on Hypertension (2002) Beyond blood pressure: the endothelium and atherosclerosis progression. Am J Hypertens 15: 115S–122S. - PubMed
-
- Davignon J, Ganz P (2004) Role of endothelial dysfunction in atherosclerosis. Circulation 109: III27–III32. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

