Autosomal dominant immune dysregulation syndrome in humans with CTLA4 mutations
- PMID: 25329329
- PMCID: PMC4668597
- DOI: 10.1038/nm.3746
Autosomal dominant immune dysregulation syndrome in humans with CTLA4 mutations
Abstract
The protein cytotoxic T lymphocyte antigen-4 (CTLA-4) is an essential negative regulator of immune responses, and its loss causes fatal autoimmunity in mice. We studied a large family in which five individuals presented with a complex, autosomal dominant immune dysregulation syndrome characterized by hypogammaglobulinemia, recurrent infections and multiple autoimmune clinical features. We identified a heterozygous nonsense mutation in exon 1 of CTLA4. Screening of 71 unrelated patients with comparable clinical phenotypes identified five additional families (nine individuals) with previously undescribed splice site and missense mutations in CTLA4. Clinical penetrance was incomplete (eight adults of a total of 19 genetically proven CTLA4 mutation carriers were considered unaffected). However, CTLA-4 protein expression was decreased in regulatory T cells (Treg cells) in both patients and carriers with CTLA4 mutations. Whereas Treg cells were generally present at elevated numbers in these individuals, their suppressive function, CTLA-4 ligand binding and transendocytosis of CD80 were impaired. Mutations in CTLA4 were also associated with decreased circulating B cell numbers. Taken together, mutations in CTLA4 resulting in CTLA-4 haploinsufficiency or impaired ligand binding result in disrupted T and B cell homeostasis and a complex immune dysregulation syndrome.
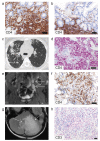
Figures



References
-
- Resnick ES, Cunningham-Rundles C. The many faces of the clinical picture of common variable immune deficiency. Curr Opin Allergy Clin Immunol. 2012;12:595–601. - PubMed
-
- Yong PF, Thaventhiran JE, Grimbacher B. “A rose is a rose is a rose,” but CVID is Not CVID common variable immune deficiency (CVID), what do we know in 2011? Adv Immunol. 2011;111:47–107. - PubMed
-
- Gathmann B, et al. Clinical picture and treatment of 2212 patients with common variable immunodeficiency. J Allergy Clin Immunol. 2014;134:116–126. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

