Long term effectiveness on prescribing of two multifaceted educational interventions: results of two large scale randomized cluster trials
- PMID: 25329386
- PMCID: PMC4201458
- DOI: 10.1371/journal.pone.0109915
Long term effectiveness on prescribing of two multifaceted educational interventions: results of two large scale randomized cluster trials
Abstract
Introduction: Information on benefits and risks of drugs is a key element affecting doctors' prescribing decisions. Outreach visits promoting independent information have proved moderately effective in changing prescribing behaviours.
Objectives: Testing the short and long-term effectiveness on general practitioners' prescribing of small groups meetings led by pharmacists.
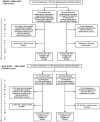
Methods: Two cluster open randomised controlled trials (RCTs) were carried out in a large scale NHS setting. Ad hoc prepared evidence based material were used considering a therapeutic area approach--TEA, with information materials on osteoporosis or prostatic hyperplasia--and a single drug oriented approach--SIDRO, with information materials on me-too drugs of 2 different classes: barnidipine or prulifloxacin. In each study, all 115 Primary Care Groups in a Northern Italy area (2.2 million inhabitants, 1737 general practitioners) were randomised to educational small groups meetings, in which available evidence was provided together with drug utilization data and clinical scenarios. Main outcomes were changes in the six-months prescription of targeted drugs. Longer term results (24 and 48 months) were also evaluated.
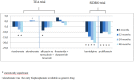
Results: In the TEA trial, one of the four primary outcomes showed a reduction (prescription of alfuzosin compared to tamsulosin and terazosin in benign prostatic hyperplasia: prescribing ratio -8.5%, p = 0.03). Another primary outcome (prescription of risedronate) showed a reduction at 24 and 48 months (-7.6%, p = 0.02; and -9,8%, p = 0.03), but not at six months (-5.1%, p = 0.36). In the SIDRO trial both primary outcomes showed a statistically significant reduction (prescription of barnidipine -9.8%, p = 0.02; prescription of prulifloxacin -11.1%, p = 0.04), which persisted or increased over time.
Interpretation: These two cluster RCTs showed the large scale feasibility of a complex educational program in a NHS setting, and its potentially relevant long-term impact on prescribing habits, in particular when focusing on a single drug. National Health systems should invest in independent drug information programs.
Trial registration: Controlled-Trials.com ISRCTN05866587.
Conflict of interest statement
Figures
References
-
- Collier J, Iheanacho I (2002) The pharmaceutical industry as an informant. Lancet 360: 1405–1409. - PubMed
-
- Ridker PM, Torres J (2006) Reported outcomes in major cardiovascular clinical trials funded by for-profit and not-for-profit organizations: 2000–2005. JAMA 295: 2270–2276. - PubMed
-
- Dickersin K (2005) Publication bias: Recognizing the problem, understanding its origins and scope, and preventing harm. In: Rothstein H, Sutton A, Borenstein M (eds). Publication bias in meta-analysis: prevention, assessment, and adjustments. London: Wiley, p 11–33.
-
- Chan AW (2008) Bias, spin, and misreporting: time for full access to trial protocols and results. PLoS Medicine 5(11): e230 DOI:10.1371/journal.pmed.0050230 - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources