The pH-responsive PacC transcription factor of Aspergillus fumigatus governs epithelial entry and tissue invasion during pulmonary aspergillosis
- PMID: 25329394
- PMCID: PMC4199764
- DOI: 10.1371/journal.ppat.1004413
The pH-responsive PacC transcription factor of Aspergillus fumigatus governs epithelial entry and tissue invasion during pulmonary aspergillosis
Erratum in
-
Correction: The pH-responsive PacC transcription factor of Aspergillus fumigatus governs epithelial entry and tissue invasion during pulmonary aspergillosis.PLoS Pathog. 2015 Apr 8;11(4):e1004802. doi: 10.1371/journal.ppat.1004802. eCollection 2015 Apr. PLoS Pathog. 2015. PMID: 25853727 Free PMC article.
-
Correction: The pH-Responsive PacC Transcription Factor of Aspergillus fumigatus Governs Epithelial Entry and Tissue Invasion during Pulmonary Aspergillosis.PLoS Pathog. 2015 Jun 18;11(6):e1004943. doi: 10.1371/journal.ppat.1004943. eCollection 2015 Jun. PLoS Pathog. 2015. PMID: 26086393 Free PMC article. No abstract available.
Abstract
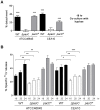
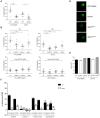
Destruction of the pulmonary epithelium is a major feature of lung diseases caused by the mould pathogen Aspergillus fumigatus. Although it is widely postulated that tissue invasion is governed by fungal proteases, A. fumigatus mutants lacking individual or multiple enzymes remain fully invasive, suggesting a concomitant requirement for other pathogenic activities during host invasion. In this study we discovered, and exploited, a novel, tissue non-invasive, phenotype in A. fumigatus mutants lacking the pH-responsive transcription factor PacC. Our study revealed a novel mode of epithelial entry, occurring in a cell wall-dependent manner prior to protease production, and via the Dectin-1 β-glucan receptor. ΔpacC mutants are defective in both contact-mediated epithelial entry and protease expression, and significantly attenuated for pathogenicity in leukopenic mice. We combined murine infection modelling, in vivo transcriptomics, and in vitro infections of human alveolar epithelia, to delineate two major, and sequentially acting, PacC-dependent processes impacting epithelial integrity in vitro and tissue invasion in the whole animal. We demonstrate that A. fumigatus spores and germlings are internalised by epithelial cells in a contact-, actin-, cell wall- and Dectin-1 dependent manner and ΔpacC mutants, which aberrantly remodel the cell wall during germinative growth, are unable to gain entry into epithelial cells, both in vitro and in vivo. We further show that PacC acts as a global transcriptional regulator of secreted molecules during growth in the leukopenic mammalian lung, and profile the full cohort of secreted gene products expressed during invasive infection. Our study reveals a combinatorial mode of tissue entry dependent upon sequential, and mechanistically distinct, perturbations of the pulmonary epithelium and demonstrates, for the first time a protective role for Dectin-1 blockade in epithelial defences. Infecting ΔpacC mutants are hypersensitive to cell wall-active antifungal agents highlighting the value of PacC signalling as a target for antifungal therapy.
Conflict of interest statement
MS conducted work on this project prior to becoming employed by Sandoz GmbH. Thus, there is no potential conflict with adherence to all the PLOS Pathogens policies on sharing data and materials. All other authors have declared that no competing interests exist.
Figures






References
-
- Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, et al. (2012) Hidden killers: human fungal infections. Sci Transl Med 4 165: 165rv13. - PubMed
-
- Kontoyiannis DP, Marr KA, Park BJ, Alexander BD, Anaissie EJ, et al. (2010) Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001-2006: overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin Infect Dis 50 8: 1091–100. - PubMed
-
- Pappas PG, Alexander BD, Andes DR, Hadley S, Kauffman CA, et al. (2010) Invasive fungal infections among organ transplant recipients: results of the Transplant-Associated Infection Surveillance Network (TRANSNET). Clin Infect Dis 50 8: 1101–11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

