Early divergence of central and peripheral neural retina precursors during vertebrate eye development
- PMID: 25329498
- PMCID: PMC4351783
- DOI: 10.1002/dvdy.24218
Early divergence of central and peripheral neural retina precursors during vertebrate eye development
Abstract
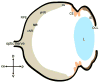
Background: During development of the vertebrate eye, optic tissue is progressively compartmentalized into functionally distinct tissues. From the central to the peripheral optic cup, the original optic neuroepithelial tissue compartmentalizes, forming retina, ciliary body, and iris. The retina can be further sub-divided into peripheral and central compartments, where the central domain is specialized for higher visual acuity, having a higher ratio and density of cone photoreceptors in most species.
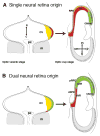
Results: Classically, models depict a segregation of the early optic cup into only two domains, neural and non-neural. Recent studies, however, uncovered discrete precursors for central and peripheral retina in the optic vesicle, indicating that the neural retina cannot be considered as a single unit with homogeneous specification and development. Instead, central and peripheral retina may be subject to distinct developmental pathways that underlie their specialization.
Conclusions: This review focuses on lineage relationships in the retina and revisits the historical context for segregation of central and peripheral retina precursors before overt eye morphogenesis.
Keywords: avian eye; fate map; lineage; optic cup; optic vesicle.
© 2014 Wiley Periodicals, Inc.
Figures


Similar articles
-
Retinal and anterior eye compartments derive from a common progenitor pool in the avian optic cup.Mol Vis. 2011;17:3347-63. Epub 2011 Dec 20. Mol Vis. 2011. PMID: 22219630 Free PMC article.
-
Central and peripheral retina arise through distinct developmental paths.PLoS One. 2013 Apr 16;8(4):e61422. doi: 10.1371/journal.pone.0061422. Print 2013. PLoS One. 2013. PMID: 23613848 Free PMC article.
-
Eye morphogenesis and patterning of the optic vesicle.Curr Top Dev Biol. 2010;93:61-84. doi: 10.1016/B978-0-12-385044-7.00003-5. Curr Top Dev Biol. 2010. PMID: 20959163 Free PMC article. Review.
-
Loss of laminin alpha 1 results in multiple structural defects and divergent effects on adhesion during vertebrate optic cup morphogenesis.Dev Biol. 2016 Aug 15;416(2):324-37. doi: 10.1016/j.ydbio.2016.06.025. Epub 2016 Jun 20. Dev Biol. 2016. PMID: 27339294 Free PMC article.
-
Build me up optic cup: Intrinsic and extrinsic mechanisms of vertebrate eye morphogenesis.Dev Biol. 2021 Aug;476:128-136. doi: 10.1016/j.ydbio.2021.03.023. Epub 2021 Mar 31. Dev Biol. 2021. PMID: 33811855 Free PMC article. Review.
Cited by
-
Angiopoietin-4-dependent venous maturation and fluid drainage in the peripheral retina.Elife. 2018 Nov 16;7:e37776. doi: 10.7554/eLife.37776. Elife. 2018. PMID: 30444491 Free PMC article.
-
Cell fate decisions, transcription factors and signaling during early retinal development.Prog Retin Eye Res. 2022 Nov;91:101093. doi: 10.1016/j.preteyeres.2022.101093. Epub 2022 Jul 8. Prog Retin Eye Res. 2022. PMID: 35817658 Free PMC article. Review.
-
Mettl14-mediated m6A modification ensures the cell-cycle progression of late-born retinal progenitor cells.Cell Rep. 2023 Jun 27;42(6):112596. doi: 10.1016/j.celrep.2023.112596. Epub 2023 Jun 1. Cell Rep. 2023. PMID: 37269288 Free PMC article.
-
BMP-induced reprogramming of the neural retina into retinal pigment epithelium requires Wnt signalling.Biol Open. 2017 Jul 15;6(7):979-992. doi: 10.1242/bio.018739. Biol Open. 2017. PMID: 28546339 Free PMC article.
-
The cellular bases of choroid fissure formation and closure.Dev Biol. 2018 Aug 15;440(2):137-151. doi: 10.1016/j.ydbio.2018.05.010. Epub 2018 May 24. Dev Biol. 2018. PMID: 29803644 Free PMC article.
References
-
- Beach DH, Jacobson M. Patterns of cell proliferation in the retina of the clawed frog during development. J Comp Neurol. 1979;183:603–613. - PubMed
-
- Belecky-Adams T, Cook B, Adler R. Correlations between terminal mitosis and differentiated fate of retinal precursor cells in vivo and in vitro: analysis with the “window-labeling” technique. Dev Biol. 1996;178:304–315. - PubMed
-
- Bellairs R, Osmond M. The atlas of chick development. San Diego: Academic Press; 2005.
-
- Bodenstein L, Sidman RL. Growth and development of the mouse retinal pigment epithelium. I. Cell and tissue morphometrics and topography of mitotic activity. Dev Biol. 1987;121:192–204. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

