Umbilical cord Wharton's jelly repeated culture system: a new device and method for obtaining abundant mesenchymal stem cells for bone tissue engineering
- PMID: 25329501
- PMCID: PMC4203828
- DOI: 10.1371/journal.pone.0110764
Umbilical cord Wharton's jelly repeated culture system: a new device and method for obtaining abundant mesenchymal stem cells for bone tissue engineering
Abstract
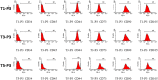
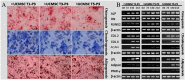
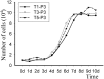
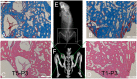
To date, various types of cells for seeding regenerative scaffolds have been used for bone tissue engineering. Among seed cells, the mesenchymal stem cells derived from human umbilical cord Wharton's jelly (hUCMSCs) represent a promising candidate and hold potential for bone tissue engineering due to the the lack of ethical controversies, accessibility, sourced by non-invasive procedures for donors, a reduced risk of contamination, osteogenic differentiation capacities, and higher immunomodulatory capacity. However, the current culture methods are somewhat complicated and inefficient and often fail to make the best use of the umbilical cord (UC) tissues. Moreover, these culture processes cannot be performed on a large scale and under strict quality control. As a result, only a small quantity of cells can be harvested using the current culture methods. To solve these problems, we designed and evaluated an UC Wharton's jelly repeated culture device. Using this device, hUCMSCs were obtained from the repeated cultures and their quantities and biological characteristics were compared. We found that using our culture device, which retained all tissue blocks on the bottom of the dish, the total number of obtained cells increased 15-20 times, and the time required for the primary passage was reduced. Moreover, cells harvested from the repeated cultures exhibited no significant difference in their immunophenotype, potential for multilineage differentiation, or proliferative, osteoinductive capacities, and final osteogenesis. The application of the repeated culture frame (RCF) not only made full use of the Wharton's jelly but also simplified and specified the culture process, and thus, the culture efficiency was significantly improved. In summary, abundant hUCMSCs of dependable quality can be acquired using the RCF.
Conflict of interest statement
Figures


Similar articles
-
Human umbilical cord Wharton's Jelly-derived mesenchymal stem cells differentiation into nerve-like cells.Chin Med J (Engl). 2005 Dec 5;118(23):1987-93. Chin Med J (Engl). 2005. PMID: 16336835
-
Characteristics of mesenchymal stem cells derived from Wharton's jelly of human umbilical cord and for fabrication of non-scaffold tissue-engineered cartilage.J Biosci Bioeng. 2014 Feb;117(2):229-235. doi: 10.1016/j.jbiosc.2013.07.001. Epub 2013 Jul 27. J Biosci Bioeng. 2014. PMID: 23899897
-
Isolation and characterization of Wharton's jelly-derived multipotent mesenchymal stromal cells obtained from bovine umbilical cord and maintained in a defined serum-free three-dimensional system.BMC Biotechnol. 2012 May 4;12:18. doi: 10.1186/1472-6750-12-18. BMC Biotechnol. 2012. PMID: 22559872 Free PMC article.
-
Wharton's Jelly stem cells: future clinical applications.Placenta. 2011 Oct;32 Suppl 4:S311-5. doi: 10.1016/j.placenta.2011.06.010. Epub 2011 Jul 6. Placenta. 2011. PMID: 21733573 Review.
-
[Research progress of biological characteristics and advantages of Wharton's jelly-mesenchymal stem cells].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2011 Jun;25(6):745-9. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2011. PMID: 21735792 Review. Chinese.
Cited by
-
Comparative analysis of human Wharton's jelly mesenchymal stem cells derived from different parts of the same umbilical cord.Cell Tissue Res. 2018 Apr;372(1):51-65. doi: 10.1007/s00441-017-2699-4. Epub 2017 Dec 4. Cell Tissue Res. 2018. PMID: 29204746 Free PMC article.
-
Wharton's jelly derived mesenchymal stem cells: future of regenerative medicine? Recent findings and clinical significance.Biomed Res Int. 2015;2015:430847. doi: 10.1155/2015/430847. Epub 2015 Mar 15. Biomed Res Int. 2015. PMID: 25861624 Free PMC article. Review.
-
Comparison of Different Culture Conditions for Mesenchymal Stem Cells from Human Umbilical Cord Wharton’s Jelly for Stem Cell Therapy.Turk J Haematol. 2020 Feb 20;37(1):67-69. doi: 10.4274/tjh.galenos.2019.2019.0439. Epub 2019 Nov 13. Turk J Haematol. 2020. PMID: 31718116 Free PMC article. No abstract available.
References
-
- Han D, Li J (2013) Repair of bone defect by using vascular bundle implantation combined with Runx II gene-transfected adipose-derived stem cells and a biodegradable matrix. Cell Tissue Res 352: 561–571. - PubMed
-
- Lee JH, Kim JH, Oh SH, Kim SJ, Hah YS, et al. (2011) Tissue-engineered bone formation using periosteal-derived cells and polydioxanone/pluronic F127 scaffold with pre-seeded adipose tissue-derived CD146 positive endothelial-like cells. Biomaterials 32: 5033–5045. - PubMed
-
- Hou T, Li Q, Luo F, Xu J, Xie Z, et al. (2010) Controlled dynamization to enhance reconstruction capacity of tissue-engineered bone in healing critically sized bone defects: an in vivo study in goats. Tissue Eng Part A 16: 201–212. - PubMed
-
- Kodama A, Kamei N, Kamei G, Kongcharoensombat W, Ohkawa S, et al. (2012) In vivo bioluminescence imaging of transplanted bone marrow mesenchymal stromal cells using a magnetic delivery system in a rat fracture model. J Bone Joint Surg Br 94: 998–1006. - PubMed
-
- Lu Z, Wang G, Dunstan CR, Chen Y, Yenn-Ru Lu W, et al. (2013) Activation and promotion of adipose stem cells by tumour necrosis factor-alpha preconditioning for bone regeneration. J Cell Physiol 228: 1737–1744. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

