Effects of the mycotoxin nivalenol on bovine articular chondrocyte metabolism in vitro
- PMID: 25329658
- PMCID: PMC4198117
- DOI: 10.1371/journal.pone.0109536
Effects of the mycotoxin nivalenol on bovine articular chondrocyte metabolism in vitro
Abstract
Objective: Kashin-Beck Disease (KBD) is an endemic, age-related degenerative osteoarthropathy and its cause is hypothesised to involve Fusarium mycotoxins. This study investigated the Fusarium mycotoxin Nivalenol (NIV) on the metabolism of bovine articular chondrocytes in vitro.
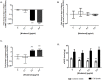
Design: The effect 0.0-0.5 µg/ml NIV on transcript levels of types I and II collagen, aggrecan, matrix metalloproteinases (MMPs), a disintegrin and metalloproteinase with thrombospondin motif (ADAMTS) and the tissue inhibitors of MMPs (TIMPs) was investigated using quantitative PCR. Amounts of sulphated glycosaminoglycans, MMPs and TIMPs were assessed using the Dimethylmethylene Blue assay, gelatin zymography and reverse gelatin zymography respectively. Cytoskeletal organisation was analysed using confocal microscopy and cytoskeletal gene and protein levels were measured by quantitative PCR and Western blot analysis, respectively.
Results: NIV caused a dose-dependent increase in aggrecan transcription with a concomitant retention of sGAG in the cell lysate. Furthermore, NIV significantly increased MMPs-2, -3 & -9, ADAMTS-4 and -5, and TIMP-2 and -3 transcript levels but inhibited type I collagen, MMP 1 and TIMP 1 mRNA levels. NIV promoted extensive cytoskeletal network remodelling, particularly with vimentin where a dose-dependent peri-nuclear aggregation occurred.
Conclusion: NIV exposure to chondrocytes decreased matrix deposition, whilst enhancing selective catabolic enzyme production, suggesting its potential for induction of cellular catabolism. This NIV-induced extracellular matrix remodelling may be due to extensive remodelling/disassembly of the cytoskeletal elements. Collectively, these findings support the hypothesis that trichothecene mycotoxins, and in particular NIV, have the potential to induce matrix catabolism and propagate the pathogenesis of KBD.
Conflict of interest statement
Figures







References
-
- Cao J, Li S, Shi Z, Yue Y, Sun J, et al. (2008) Articular cartilage metabolism in patients with Kashin-Beck Disease: an endemic osteoarthropathy in China. Osteoarthritis Cartilage 16: 680–688. - PubMed
-
- Li FS, Duan YJ, Yan SJ, Guan JY, Zou LM, et al. (1990) Presenile (early ageing) changes in tissues of Kaschin-Beck disease and its pathogenetic significance. Mech Ageing Dev 54: 103–120. - PubMed
-
- Zhang JB, Li HP, Dang FJ, Qu B, Xu YB, et al. (2007) Determination of the trichothecene mycotoxin chemotypes and associated geographical distribution and phylogenetic species of the Fusarium graminearum clade from China. Mycol Res 111: 967–975. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

