PLK1 and β-TrCP-dependent ubiquitination and degradation of Rap1GAP controls cell proliferation
- PMID: 25329897
- PMCID: PMC4201484
- DOI: 10.1371/journal.pone.0110296
PLK1 and β-TrCP-dependent ubiquitination and degradation of Rap1GAP controls cell proliferation
Abstract
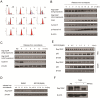
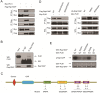
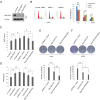
Rap1GAP is a GTPase-activating protein (GAP) that specifically stimulates the GTP hydrolysis of Rap1 GTPase. Although Rap1GAP is recognized as a tumor suppressor gene and downregulated in various cancers, little is known regarding the regulation of Rap1GAP ubiquitination and degradation under physiological conditions. Here, we demonstrated that Rap1GAP is ubiquitinated and degraded through proteasome pathway in mitosis. Proteolysis of Rap1GAP requires the PLK1 kinase and β-TrCP ubiquitin ligase complex. We revealed that PLK1 interacts with Rap1GAP in vivo through recognition of an SSP motif within Rap1GAP. PLK1 phosphorylates Ser525 in conserved 524DSGHVS529 degron of Rap1GAP and promotes its interaction with β-TrCP. We also showed that Rap1GAP was a cell cycle regulator and that tight regulation of the Rap1GAP degradation in mitosis is required for cell proliferation.
Conflict of interest statement
Figures


Similar articles
-
Plk1- and beta-TrCP-dependent degradation of Bora controls mitotic progression.J Cell Biol. 2008 Apr 7;181(1):65-78. doi: 10.1083/jcb.200712027. Epub 2008 Mar 31. J Cell Biol. 2008. PMID: 18378770 Free PMC article.
-
G1/S phase progression is regulated by PLK1 degradation through the CDK1/βTrCP axis.FASEB J. 2017 Jul;31(7):2925-2936. doi: 10.1096/fj.201601108R. Epub 2017 Mar 30. FASEB J. 2017. PMID: 28360195
-
Cyclin-dependent kinase (CDK) phosphorylation destabilizes somatic Wee1 via multiple pathways.Proc Natl Acad Sci U S A. 2005 Aug 16;102(33):11663-8. doi: 10.1073/pnas.0500410102. Epub 2005 Aug 5. Proc Natl Acad Sci U S A. 2005. PMID: 16085715 Free PMC article.
-
Regulation of NF-κB by ubiquitination and degradation of the IκBs.Immunol Rev. 2012 Mar;246(1):77-94. doi: 10.1111/j.1600-065X.2012.01098.x. Immunol Rev. 2012. PMID: 22435548 Review.
-
The Mitotic Cancer Target Polo-Like Kinase 1: Oncogene or Tumor Suppressor?Genes (Basel). 2019 Mar 11;10(3):208. doi: 10.3390/genes10030208. Genes (Basel). 2019. PMID: 30862113 Free PMC article. Review.
Cited by
-
Casein kinase 1α mediates phosphorylation of the Merkel cell polyomavirus large T antigen for β-TrCP destruction complex interaction and subsequent degradation.mBio. 2024 Aug 14;15(8):e0111724. doi: 10.1128/mbio.01117-24. Epub 2024 Jun 28. mBio. 2024. PMID: 38940554 Free PMC article.
-
EBV-miR-BART10-3p facilitates epithelial-mesenchymal transition and promotes metastasis of nasopharyngeal carcinoma by targeting BTRC.Oncotarget. 2015 Dec 8;6(39):41766-82. doi: 10.18632/oncotarget.6155. Oncotarget. 2015. PMID: 26497204 Free PMC article.
-
Mex3a promotes oncogenesis through the RAP1/MAPK signaling pathway in colorectal cancer and is inhibited by hsa-miR-6887-3p.Cancer Commun (Lond). 2021 Jun;41(6):472-491. doi: 10.1002/cac2.12149. Epub 2021 Feb 27. Cancer Commun (Lond). 2021. PMID: 33638620 Free PMC article.
-
Downregulation of Rap1Gap: A Switch from DCIS to Invasive Breast Carcinoma via ERK/MAPK Activation.Neoplasia. 2018 Sep;20(9):951-963. doi: 10.1016/j.neo.2018.07.002. Epub 2018 Aug 22. Neoplasia. 2018. PMID: 30144784 Free PMC article.
-
Transcriptomic Properties of HER2+ Ductal Carcinoma In Situ of the Breast Associate with Absence of Immune Cells.Biology (Basel). 2021 Aug 12;10(8):768. doi: 10.3390/biology10080768. Biology (Basel). 2021. PMID: 34440000 Free PMC article.
References
-
- Bos JL, de Rooij J, Reedquist KA (2001) Rap1 signalling: adhering to new models. Nat Rev Mol Cell Biol 2: 369–377. - PubMed
-
- Zheng H, Gao L, Feng Y, Yuan L, Zhao H, et al. (2009) Down-regulation of Rap1GAP via promoter hypermethylation promotes melanoma cell proliferation, survival, and migration. Cancer Res 69: 449–457. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

