Upregulation of GPR109A in Parkinson's disease
- PMID: 25329911
- PMCID: PMC4201464
- DOI: 10.1371/journal.pone.0109818
Upregulation of GPR109A in Parkinson's disease
Abstract
Background: Anecdotal animal and human studies have implicated the symptomatic and neuroprotective roles of niacin in Parkinson's disease (PD). Niacin has a high affinity for GPR109A, an anti-inflammatory receptor. Niacin is also thought to be involved in the regulation of circadian rhythm. Here we evaluated the relationships among the receptor, niacin levels and EEG night-sleep in individuals with PD.
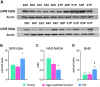
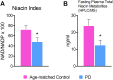
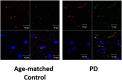
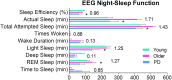
Methods and findings: GPR109A expression (blood and brain), niacin index (NAD-NADP ratio) and cytokine markers (blood) were analyzed. Measures of night-sleep function (EEG) and perceived sleep quality (questionnaire) were assessed. We observed significant up-regulation of GPR109A expression in the blood as well as in the substantia nigra (SN) in the PD group compared to age-matched controls. Confocal microscopy demonstrated co-localization of GPR109A staining with microglia in PD SN. Pro and anti-inflammatory cytokines did not show significant differences between the groups; however IL1-β, IL-4 and IL-7 showed an upward trend in PD. Time to sleep (sleep latency), EEG REM and sleep efficiency were different between PD and age-matched controls. Niacin levels were lower in PD and were associated with increased frequency of experiencing body pain and decreased duration of deep sleep.
Conclusions: The findings of associations among the GPR109A receptor, niacin levels and night-sleep function in individuals with PD are novel. Further studies are needed to understand the pathophysiological mechanisms of action of niacin, GPR109A expression and their associations with night-sleep function. It would be also crucial to study GPR109A expression in neurons, astrocytes, and microglia in PD. A clinical trial to determine the symptomatic and/or neuroprotective effect of niacin supplementation is warranted.
Conflict of interest statement
Figures

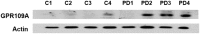
References
-
- Barnum CJ, Tansey MG (2010) Modeling neuroinflammatory pathogenesis of Parkinson's disease. Progress in Brain Research 184: 113–132. - PubMed
-
- Banati RB, Daniel SE, Blunt SB (1998) Glial pathology but absence of apoptotic nigral neurons in long-standing Parkinson's disease. Movement Disorders 13: 221–227. - PubMed
-
- Crotty S, Fitzgerald P, Tuohy E, Harris DM, Fisher A, et al. (2008) Neuroprotective effects of novel phosphatidylglycerol-based phospholipids in the 6-hydroxydopamine model of Parkinson's disease. European Journal of Neuroscience 27: 294–300. - PubMed
-
- Maciejewski-Lenoir D, Richman JG, Hakak Y, Gaidarov I, Behan DP, et al. (2006) Langerhans cells release prostaglandin D2 in response to nicotinic acid. Journal of Investigative Dermatology 126: 2637–2646. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

