Myosins VIII and XI play distinct roles in reproduction and transport of tobacco mosaic virus
- PMID: 25329993
- PMCID: PMC4199776
- DOI: 10.1371/journal.ppat.1004448
Myosins VIII and XI play distinct roles in reproduction and transport of tobacco mosaic virus
Abstract
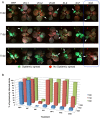
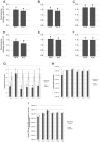
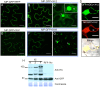
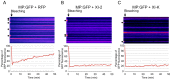
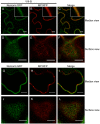
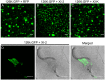
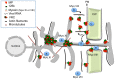
Viruses are obligatory parasites that depend on host cellular factors for their replication as well as for their local and systemic movement to establish infection. Although myosin motors are thought to contribute to plant virus infection, their exact roles in the specific infection steps have not been addressed. Here we investigated the replication, cell-to-cell and systemic spread of Tobacco mosaic virus (TMV) using dominant negative inhibition of myosin activity. We found that interference with the functions of three class VIII myosins and two class XI myosins significantly reduced the local and long-distance transport of the virus. We further determined that the inactivation of myosins XI-2 and XI-K affected the structure and dynamic behavior of the ER leading to aggregation of the viral movement protein (MP) and to a delay in the MP accumulation in plasmodesmata (PD). The inactivation of myosin XI-2 but not of myosin XI-K affected the localization pattern of the 126k replicase subunit and the level of TMV accumulation. The inhibition of myosins VIII-1, VIII-2 and VIII-B abolished MP localization to PD and caused its retention at the plasma membrane. These results suggest that class XI myosins contribute to the viral propagation and intracellular trafficking, whereas myosins VIII are specifically required for the MP targeting to and virus movement through the PD. Thus, TMV appears to recruit distinct myosins for different steps in the cell-to-cell spread of the infection.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

