Quantiferon-TB Gold: performance for ruling out active tuberculosis in HIV-infected adults with high CD4 count in Côte d'Ivoire, West Africa
- PMID: 25330161
- PMCID: PMC4199568
- DOI: 10.1371/journal.pone.0107245
Quantiferon-TB Gold: performance for ruling out active tuberculosis in HIV-infected adults with high CD4 count in Côte d'Ivoire, West Africa
Abstract
Objective: To assess the performance of QuantiFERON-TB Gold In-Tube (QFT-GIT) test for active tuberculosis (TB) in HIV adults, and its variation over time in patients on antiretroviral therapy (ART) and/or isoniazide preventive therapy (IPT).
Methods: Transversal study and cohort nested in the Temprano ANRS 12136 randomized controlled trial assessing benefits of initiating ART earlier than currently recommended by World Health Organization, with or without a 6-month IPT. Performance of QFT-GIT for detecting active TB at baseline in the first 50% participants, and 12-month incidence of conversion/reversion in the first 25% participants were assessed. QFT-GIT threshold for positivity was 0.35 IU/ml.
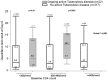
Results: Among the 975 first participants (median baseline CD4 count 383/mm3, positive QFT-GIT test 35%), 2.7% had active TB at baseline. QFT-GIT sensitivity, specificity, positive and negative predictive value for active TB were 88.0%, 66.6%, 6.5% and 99.5%. For the 444 patients with a second test at 12 months, rates for conversion and reversion were 9.3% and 14%. Reversion was more frequent in patients without ART and younger patients. IPT and early ART were not associated with reversion/conversion.
Conclusion: A negative QFT-GIT could rule out active TB in HIV-infected adults not severely immunosuppressed, thus avoiding repeated TB testing and accelerating diagnosis and care for other diseases.
Trial registration: ClinicalTrials.gov NCT00495651.
Conflict of interest statement
Figures

References
-
- World Health Organization (2013) Global tuberculosis report: Available: http://apps.who.int/iris/bitstream/10665/91355/1/9789241564656_eng.pdf. Accessed 2014 January 21.
-
- Moh R, Danel C, Messou E, Ouassa T, Gabillard D, et al. (2007) Incidence and determinants of mortality and morbidity following early antiretroviral therapy initiation in HIV-infected adults in West Africa. AIDS 21: 2483–91. - PubMed
-
- Fitzgerald DW, Desvarieux M, Severe P, Joseph P, Johnson WD, et al. (2000) Effect of post-treatment isoniazid on prevention of recurrent tuberculosis in HIV-1-infected individuals: a randomised trial. Lancet 356: 1470–4. - PubMed
-
- Hawken MP, Meme HK, Elliott LC, Chakaya JM, Morris JS, et al. (1997) Isoniazid preventive therapy for tuberculosis in HIV-1-infected adults: results of a randomized controlled trial. AIDS 11: 875–82. - PubMed
-
- Bucher HC, Griffith LE, Guyatt GH, Sudre P, Naef M, et al. (1999) Isoniazid prophylaxis for tuberculosis in HIV infection: a meta-analysis of randomized controlled trials. AIDS 13: 501–7. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

