Redox regulation of NLRP3 inflammasomes: ROS as trigger or effector?
- PMID: 25330206
- PMCID: PMC4403231
- DOI: 10.1089/ars.2014.5994
Redox regulation of NLRP3 inflammasomes: ROS as trigger or effector?
Abstract
Significance: Inflammasomes are multiprotein complexes localized within the cytoplasm of the cell that are responsible for the maturation of proinflammatory cytokines such as interleukin-1β (IL-1β) and IL-18, and the activation of a highly inflammatory form of cell death, pyroptosis. In response to infection or cellular stress, inflammasomes are assembled, activated, and involved in host defense and pathophysiology of diseases. Clarification of the molecular mechanisms leading to the activation of this intracellular inflammatory machinery may provide new insights into the concept of inflammation as the root of and route to human diseases.
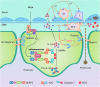
Recent advances: The activation of inflammasomes, specifically the most fully characterized inflammasome-the nucleotide-binding oligomerization domain (NOD)-like receptor containing pyrin domain 3 (NLRP3) inflammasome, is now emerging as a critical molecular mechanism for many degenerative diseases. Several models have been developed to describe how NLRP3 inflammasomes are activated, including K(+) efflux, lysosome function, endoplasmic reticulum (ER) stress, intracellular calcium, ubiquitination, microRNAs, and, in particular, reactive oxygen species (ROS).
Critical issues: ROS may serve as a "kindling" or triggering factor to activate NLRP3 inflammasomes as well as "bonfire" or "effector" molecules, resulting in pathological processes. Increasing evidence seeks to understand how this spatiotemporal action of ROS occurs during NLRP3 inflammasome activation, which will be a major focus of this review.
Future directions: It is imperative to know how this dual action of ROS works during NLRP3 inflammation activation on different stimuli and what relevance such spatiotemporal redox regulation of NLRP3 inflammasomes has in cell or organ functions and possible human diseases.
Figures






References
-
- Abais JM, Xia M, Boini KM, and Li PL. Contribution of guanine nucleotide exchange factor Vav2 to homocysteine-induced NLRP3 inflammasome activation in mouse podocytes. FASEB J 28: 1063.6, 2014
-
- Aganna E, Martinon F, Hawkins PN, Ross JB, Swan DC, Booth DR, Lachmann HJ, Bybee A, Gaudet R, Woo P, Feighery C, Cotter FE, Thome M, Hitman GA, Tschopp J, and McDermott MF. Association of mutations in the NALP3/CIAS1/PYPAF1 gene with a broad phenotype including recurrent fever, cold sensitivity, sensorineural deafness, and AA amyloidosis. Arthritis Rheum 46: 2445–2452, 2002 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
