Crovirin, a snake venom cysteine-rich secretory protein (CRISP) with promising activity against Trypanosomes and Leishmania
- PMID: 25330220
- PMCID: PMC4199522
- DOI: 10.1371/journal.pntd.0003252
Crovirin, a snake venom cysteine-rich secretory protein (CRISP) with promising activity against Trypanosomes and Leishmania
Abstract
Background: The neglected human diseases caused by trypanosomatids are currently treated with toxic therapy with limited efficacy. In search for novel anti-trypanosomatid agents, we showed previously that the Crotalus viridis viridis (Cvv) snake venom was active against infective forms of Trypanosoma cruzi. Here, we describe the purification of crovirin, a cysteine-rich secretory protein (CRISP) from Cvv venom with promising activity against trypanosomes and Leishmania.
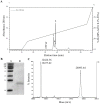
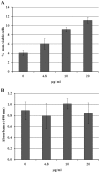
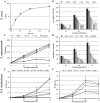
Methodology/principal findings: Crude venom extract was loaded onto a reverse phase analytical (C8) column using a high performance liquid chromatographer. A linear gradient of water/acetonitrile with 0.1% trifluoroacetic acid was used. The peak containing the isolated protein (confirmed by SDS-PAGE and mass spectrometry) was collected and its protein content was measured. T. cruzi trypomastigotes and amastigotes, L. amazonensis promastigotes and amastigotes and T. brucei rhodesiense procyclic and bloodstream trypomastigotes were challenged with crovirin, whose toxicity was tested against LLC-MK2 cells, peritoneal macrophages and isolated murine extensor digitorum longus muscle. We purified a single protein from Cvv venom corresponding, according to Nano-LC MS/MS sequencing, to a CRISP of 24,893.64 Da, henceforth referred to as crovirin. Human infective trypanosomatid forms, including intracellular amastigotes, were sensitive to crovirin, with low IC50 or LD50 values (1.10-2.38 µg/ml). A considerably higher concentration (20 µg/ml) of crovirin was required to elicit only limited toxicity on mammalian cells.
Conclusions: This is the first report of CRISP anti-protozoal activity, and suggests that other members of this family might have potential as drugs or drug leads for the development of novel agents against trypanosomatid-borne neglected diseases.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Alvar J, Croft S, Olliaro P (2006) Chemotherapy in the treatment and control of leishmaniasis. Adv Parasitol 61: 223–274. - PubMed
-
- Schmunis GA, Yadon ZE (2010) Chagas disease: A Latin American health problem becoming a world health problem. Acta Trop 115: 15–21. - PubMed
-
- Utzinger J, Becker SL, Knopp S, Blum J, Neumayr AL, et al. (2012) Neglected tropical diseases: diagnosis, clinical management, treatment and control. Swiss Medical Weekly 142: w13727. - PubMed
-
- Don R, Chatelain E (2009) Drug Discovery for Neglected Diseases: View of A Public-Private Partnership. In: Selzer PM, editor. Antiparasitic and Antibacterial Drug Discovery: From Molecular Targets to Drug Candidates. pp. 33–43.
-
- Rodrigues JC, Godinho JL, de Souza W (2014) Biology of human pathogenic trypanosomatids: epidemiology, lifecycle and ultrastructure. Subcell Biochem 74: 1–42. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

