Role of TRPV1 channels in ischemia/reperfusion-induced acute kidney injury
- PMID: 25330307
- PMCID: PMC4201466
- DOI: 10.1371/journal.pone.0109842
Role of TRPV1 channels in ischemia/reperfusion-induced acute kidney injury
Erratum in
- PLoS One. 2014;9(12):e115469
Abstract
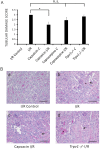
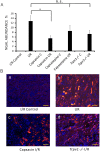
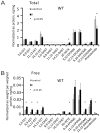
Objectives: Transient receptor potential vanilloid 1 (TRPV1) -positive sensory nerves are widely distributed in the kidney, suggesting that TRPV1-mediated action may participate in the regulation of renal function under pathophysiological conditions. Stimulation of TRPV1 channels protects against ischemia/reperfusion (I/R)-induced acute kidney injury (AKI). However, it is unknown whether inhibition of these channels is detrimental in AKI or not. We tested the role of TRPV1 channels in I/R-induced AKI by modulating these channels with capsaicin (TRPV1 agonist), capsazepine (TRPV1 antagonist) and using Trpv1-/- mice.
Methods and results: Anesthetized C57BL/6 mice were subjected to 25 min of renal ischemia and 24 hrs of reperfusion. Mice were pretreated with capsaicin (0.3 mg/kg body weight) or capsazepine (50 mg/kg body weight). Capsaicin ameliorated the outcome of AKI, as measured by serum creatinine levels, tubular damage,neutrophil gelatinase-associated lipocalin (NGAL) abundance and Ly-6B.2 positive polymorphonuclear inflammatory cells in injured kidneys. Neither capsazepine nor deficiency of TRPV1 did deteriorate renal function or histology after AKI. Measurements of endovanilloids in kidney tissue indicate that 20-hydroxyeicosatetraeonic acid (20-HETE) or epoxyeicosatrienoic acids (EETs) are unlikely involved in the beneficial effects of capsaicin on I/R-induced AKI.
Conclusions: Activation of TRPV1 channels ameliorates I/R-induced AKI, but inhibition of these channels does not affect the outcome of AKI. Our results may have clinical implications for long-term safety of renal denervation to treat resistant hypertension in man, with respect to the function of primary sensory nerves in the response of the kidney to ischemic stimuli.
Conflict of interest statement
Figures



References
-
- Barajas L, Liu L, Powers K (1992) Anatomy of the renal innervation: intrarenal aspects and ganglia of origin. Can J Physiol Pharmacol 70: 735–749 Available: http://www.ncbi.nlm.nih.gov/pubmed/1423018 Accessed 1 April 2014. - PubMed
-
- Ditting T, Freisinger W, Siegel K, Fiedler C, Small L, et al. (2012) Tonic postganglionic sympathetic inhibition induced by afferent renal nerves? Hypertension 59: 467–476 Available: http://www.ncbi.nlm.nih.gov/pubmed/22215714 Accessed 2014 April 1. - PubMed
-
- Wang Y, Chen AF, Wang DH (2005) ET(A) receptor blockade prevents renal dysfunction in salt-sensitive hypertension induced by sensory denervation. Am J Physiol Heart Circ Physiol 289: H2005–11 Available: http://www.ncbi.nlm.nih.gov/pubmed/15994858 Accessed 1 April 2014. - PubMed
-
- Xie C, Wang DH (2010) Effects of a high-salt diet on TRPV-1-dependent renal nerve activity in Dahl salt-sensitive rats. Am J Nephrol 32: 194–200 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2980518&tool=p... Accessed 2014 April 1. - PMC - PubMed
-
- Yang D, Luo Z, Ma S, Wong WT, Ma L, et al. (2010) Activation of TRPV1 by dietary capsaicin improves endothelium-dependent vasorelaxation and prevents hypertension. Cell Metab 12: 130–141 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3906919&tool=p... Accessed 2014 April 1. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

