Altered theca and cumulus oocyte complex gene expression, follicular arrest and reduced fertility in cows with dominant follicle follicular fluid androgen excess
- PMID: 25330369
- PMCID: PMC4199720
- DOI: 10.1371/journal.pone.0110683
Altered theca and cumulus oocyte complex gene expression, follicular arrest and reduced fertility in cows with dominant follicle follicular fluid androgen excess
Abstract
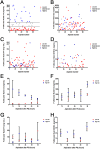
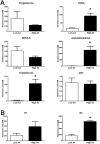
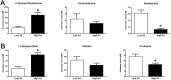
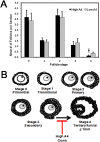
Aspiration of bovine follicles 12-36 hours after induced corpus luteum lysis serendipitously identified two populations of cows, one with High androstenedione (A4; >40 ng/ml; mean = 102) and another with Low A4 (<20 ng/ml; mean = 9) in follicular fluid. We hypothesized that the steroid excess in follicular fluid of dominant follicles in High A4 cows would result in reduced fertility through altered follicle development and oocyte maternal RNA abundance. To test this hypothesis, estrous cycles of cows were synchronized and ovariectomy was performed 36 hours later. HPLC MS/MS analysis of follicular fluid showed increased dehydroepiandrosterone (6-fold), A4 (158-fold) and testosterone (31-fold) in the dominant follicle of High A4 cows. However, estrone (3-fold) and estradiol (2-fold) concentrations were only slightly elevated, suggesting a possible inefficiency in androgen to estrogen conversion in High A4 cows. Theca cell mRNA expression of LHCGR, GATA6, CYP11A1, and CYP17A1 was greater in High A4 cows. Furthermore, abundance of ZAR1 was decreased 10-fold in cumulus oocyte complexes from High A4 cows, whereas NLRP5 abundance tended to be 19.8-fold greater (P = 0.07). There was a tendency for reduction in stage 4 follicles in ovarian cortex samples from High A4 cows suggesting that progression to antral stages were impaired. High A4 cows tended (P<0.07) to have a 17% reduction in calving rate compared with Low A4 cows suggesting reduced fertility in the High A4 population. These data suggest that the dominant follicle environment of High A4 cows including reduced estrogen conversion and androgen excess contributes to infertility in part through altered follicular and oocyte development.
Conflict of interest statement
Figures


Similar articles
-
Sheep with ovarian androgen excess have fibrosis and follicular arrest with increased mRNA abundance for steroidogenic enzymes and gonadotropin receptors.J Anim Sci. 2023 Jan 3;101:skad082. doi: 10.1093/jas/skad082. J Anim Sci. 2023. PMID: 37061806 Free PMC article.
-
Levels of insulin-like growth factor (IGF) binding proteins, luteinizing hormone and IGF-I receptors, and steroids in dominant follicles during the first follicular wave in cattle exhibiting regular estrous cycles.Endocrinology. 1996 Jul;137(7):2842-50. doi: 10.1210/endo.137.7.8770905. Endocrinology. 1996. PMID: 8770905
-
Naturally occurring androgen excess cows are present in dairy and beef herds and have similar characteristics to women with PCOS.J Anim Sci. 2022 Jun 1;100(6):skac151. doi: 10.1093/jas/skac151. J Anim Sci. 2022. PMID: 35648128 Free PMC article.
-
Ovarian follicular and luteal physiology.Int Rev Physiol. 1980;22:117-201. Int Rev Physiol. 1980. PMID: 6248477 Review.
-
Development of the dominant follicle: mechanisms of selection and maintenance of oocyte quality.Soc Reprod Fertil Suppl. 2007;64:141-63. doi: 10.5661/rdr-vi-141. Soc Reprod Fertil Suppl. 2007. PMID: 17491145 Review.
Cited by
-
Attainment and maintenance of pubertal cyclicity may predict reproductive longevity in beef heifers†.Biol Reprod. 2021 Jun 4;104(6):1360-1372. doi: 10.1093/biolre/ioab044. Biol Reprod. 2021. PMID: 33709137 Free PMC article.
-
Beef cows with atypical estrous cyclicity at puberty produced calves with deficits in preweaning muscling, metabolic indicators, and myoblast function but not in feedlot performance.Transl Anim Sci. 2020 Dec 22;4(Suppl 1):S127-S131. doi: 10.1093/tas/txaa119. eCollection 2020 Dec. Transl Anim Sci. 2020. PMID: 33381735 Free PMC article. No abstract available.
-
Formation and characterization of lipid droplets of the bovine corpus luteum.Sci Rep. 2020 Jul 9;10(1):11287. doi: 10.1038/s41598-020-68091-2. Sci Rep. 2020. PMID: 32647143 Free PMC article.
-
Gene expression profiling of bovine ovarian follicular and luteal cells provides insight into cellular identities and functions.Mol Cell Endocrinol. 2017 Jan 5;439:379-394. doi: 10.1016/j.mce.2016.09.029. Epub 2016 Sep 28. Mol Cell Endocrinol. 2017. PMID: 27693538 Free PMC article.
-
Hyperandrogenism associated with an ovarian remnant in a spayed female cat.JFMS Open Rep. 2019 Nov 14;5(2):2055116919885698. doi: 10.1177/2055116919885698. eCollection 2019 Jul-Dec. JFMS Open Rep. 2019. PMID: 31763051 Free PMC article.
References
-
- Conley AJ, Bird IM (1997) The role of cytochrome P450 17 alpha-hydroxylase and 3 beta-hydroxysteroid dehydrogenase in the integration of gonadal and adrenal steroidogenesis via the delta 5 and delta 4 pathways of steroidogenesis in mammals. Biol Reprod 56: 789–799. - PubMed
-
- Stocco DM, Clark BJ (1996) Regulation of the acute production of steroids in steroidogenic cells. Endocr Rev 17: 221–244. - PubMed
-
- Miller WL (2007) StAR search–what we know about how the steroidogenic acute regulatory protein mediates mitochondrial cholesterol import. Mol Endocrinol 21: 589–601. - PubMed
-
- Richards JS (1994) Hormonal control of gene expression in the ovary. Endocr Rev 15: 725–751. - PubMed
-
- Lavoie HA, King SR (2009) Transcriptional regulation of steroidogenic genes: STARD1, CYP11A1 and HSD3B. Exp Biol Med (Maywood) 234: 880–907. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

