A molecular fluorescent probe for targeted visualization of temperature at the endoplasmic reticulum
- PMID: 25330751
- PMCID: PMC4204065
- DOI: 10.1038/srep06701
A molecular fluorescent probe for targeted visualization of temperature at the endoplasmic reticulum
Abstract
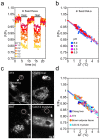
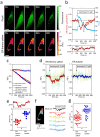
The dynamics of cellular heat production and propagation remains elusive at a subcellular level. Here we report the first small molecule fluorescent thermometer selectively targeting the endoplasmic reticulum (ER thermo yellow), with the highest sensitivity reported so far (3.9%/°C). Unlike nanoparticle thermometers, ER thermo yellow stains the target organelle evenly without the commonly encountered problem of aggregation, and successfully demonstrates the ability to monitor intracellular temperature gradients generated by external heat sources in various cell types. We further confirm the ability of ER thermo yellow to monitor heat production by intracellular Ca(2+) changes in HeLa cells. Our thermometer anchored at nearly-zero distance from the ER, i.e. the heat source, allowed the detection of the heat as it readily dissipated, and revealed the dynamics of heat production in real time at a subcellular level.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

