Structural determinants of host specificity of complement Factor H recruitment by Streptococcus pneumoniae
- PMID: 25330773
- PMCID: PMC5600146
- DOI: 10.1042/BJ20141069
Structural determinants of host specificity of complement Factor H recruitment by Streptococcus pneumoniae
Abstract
Many human pathogens have strict host specificity, which affects not only their epidemiology but also the development of animal models and vaccines. Complement Factor H (FH) is recruited to pneumococcal cell surface in a human-specific manner via the N-terminal domain of the pneumococcal protein virulence factor choline-binding protein A (CbpAN). FH recruitment enables Streptococcus pneumoniae to evade surveillance by human complement system and contributes to pneumococcal host specificity. The molecular determinants of host specificity of complement evasion are unknown. In the present study, we show that a single human FH (hFH) domain is sufficient for tight binding of CbpAN, present the crystal structure of the complex and identify the critical structural determinants for host-specific FH recruitment. The results offer new approaches to the development of better animal models for pneumococcal infection and redesign of the virulence factor for pneumococcal vaccine development and reveal how FH recruitment can serve as a mechanism for both pneumococcal complement evasion and adherence.
Figures
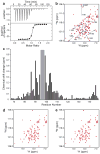
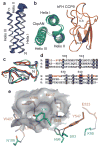

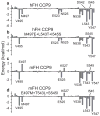
References
-
- Serruto D, Rappuoli R, Scarselli M, Gros P, van Strijp JAG. Molecular mechanisms of complement evasion: learning from staphylococci and meningococci. Nature Rev Microbiol. 2010;8:393–399. - PubMed
-
- Zipfel PF, Skerka C. Complement regulators and inhibitory proteins. Nat Rev Immunol. 2009;9:729–740. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

