Regulated apoptosis of genetically modified hematopoietic stem and progenitor cells via an inducible caspase-9 suicide gene in rhesus macaques
- PMID: 25330775
- PMCID: PMC4270878
- DOI: 10.1002/stem.1869
Regulated apoptosis of genetically modified hematopoietic stem and progenitor cells via an inducible caspase-9 suicide gene in rhesus macaques
Abstract
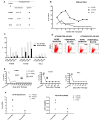
The high risk of insertional oncogenesis reported in clinical trials using integrating retroviral vectors to genetically modify hematopoietic stem and progenitor cells (HSPCs) requires the development of safety strategies to minimize risks associated with novel cell and gene therapies. The ability to ablate genetically modified cells in vivo is desirable, should an abnormal clone emerge. Inclusion of "suicide genes" in vectors to facilitate targeted ablation of vector-containing abnormal clones in vivo is one potential safety approach. We tested whether the inclusion of the "inducible Caspase-9" (iCasp9) suicide gene in a gamma-retroviral vector facilitated efficient elimination of vector-containing HSPCs and their hematopoietic progeny in vivo long-term, in an autologous non-human primate transplantation model. Following stable engraftment of iCasp9 expressing hematopoietic cells in rhesus macaques, administration of AP1903, a chemical inducer of dimerization able to activate iCasp9, specifically eliminated vector-containing cells in all hematopoietic lineages long-term, suggesting activity at the HSPC level. Between 75% and 94% of vector-containing cells were eliminated by well-tolerated AP1903 dosing, but lack of complete ablation was linked to lower iCasp9 expression in residual cells. Further investigation of resistance mechanisms demonstrated upregulation of Bcl-2 in hematopoietic cell lines transduced with the vector and resistant to AP1903 ablation. These results demonstrate both the potential and the limitations of safety approaches using iCasp9 to HSPC-targeted gene therapy settings, in a model with great relevance to clinical development.
Keywords: Gene therapy; Genotoxicity; HSC transplantation; Suicide gene; iCasp9.
© 2014 AlphaMed Press.
Conflict of interest statement
No other authors have relevant conflicts of interest.
Figures



References
-
- Hacein-Bey-Abina S, Garrigue A, Wang GP, Soulier J, Lim A, Morillon E, Clappier E, Caccavelli L, Delabesse E, Beldjord K, Asnafi V, MacIntyre E, Dal Cortivo L, Radford I, Brousse N, Sigaux F, Moshous D, Hauer J, Borkhardt A, Belohradsky BH, Wintergerst U, Velez MC, Leiva L, Sorensen R, Wulffraat N, Blanche S, Bushman FD, Fischer A, Cavazzana-Calvo M. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of scid-x1. J Clin Invest. 2008;118:3132–3142. - PMC - PubMed
-
- Hacein-Bey-Abina S, Von Kalle C, Schmidt M, McCormack MP, Wulffraat N, Leboulch P, Lim A, Osborne CS, Pawliuk R, Morillon E, Sorensen R, Forster A, Fraser P, Cohen JI, de Saint Basile G, Alexander I, Wintergerst U, Frebourg T, Aurias A, Stoppa-Lyonnet D, Romana S, Radford-Weiss I, Gross F, Valensi F, Delabesse E, Macintyre E, Sigaux F, Soulier J, Leiva LE, Wissler M, Prinz C, Rabbitts TH, Le Deist F, Fischer A, Cavazzana-Calvo M. Lmo2-associated clonal t cell proliferation in two patients after gene therapy for scid-x1. Science. 2003;302:415–419. - PubMed
-
- Howe SJ, Mansour MR, Schwarzwaelder K, Bartholomae C, Hubank M, Kempski H, Brugman MH, Pike-Overzet K, Chatters SJ, de Ridder D, Gilmour KC, Adams S, Thornhill SI, Parsley KL, Staal FJ, Gale RE, Linch DC, Bayford J, Brown L, Quaye M, Kinnon C, Ancliff P, Webb DK, Schmidt M, von Kalle C, Gaspar HB, Thrasher AJ. Insertional mutagenesis combined with acquired somatic mutations causes leukemogenesis following gene therapy of scid-x1 patients. The Journal of clinical investigation. 2008;118:3143–3150. - PMC - PubMed
-
- Stein S, Ott MG, Schultze-Strasser S, Jauch A, Burwinkel B, Kinner A, Schmidt M, Kramer A, Schwable J, Glimm H, Koehl U, Preiss C, Ball C, Martin H, Gohring G, Schwarzwaelder K, Hofmann WK, Karakaya K, Tchatchou S, Yang R, Reinecke P, Kuhlcke K, Schlegelberger B, Thrasher AJ, Hoelzer D, Seger R, von Kalle C, Grez M. Genomic instability and myelodysplasia with monosomy 7 consequent to evi1 activation after gene therapy for chronic granulomatous disease. Nature medicine. 2010;16:198–204. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

