The regulation of limb stiffness in the context of locomotor tasks
- PMID: 25330884
- PMCID: PMC9806734
- DOI: 10.1007/978-1-4939-1338-1_4
The regulation of limb stiffness in the context of locomotor tasks
Abstract
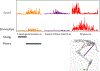
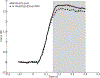
Locomotion on ramped surfaces requires modulation of both pattern generating circuits and limb stiffness. In order to meet the mechanical demands of locomotion under these conditions, muscular activation patterns must correspond to the appropriate functions, whether the muscles are serving as force generators or brakes. Limb stiffness is a critical mechanical property that determines how the body interacts with the environment, and is regulated by both intrinsic and neural mechanisms. We have recently investigated how pattern generation, stiffness and proprioceptive feedback are modulated in a task specific way using the decerebrate cat preparation. Our results confirm previous research using intact animals that during level and upslope walking, hip and ankle extensors are recruited for propulsion during stance. During downslope walking, hip extensors are inhibited and hip flexors are recruited during stance to provide the needed braking action. Our new data further show that endpoint stiffness of the limb is correspondingly reduced for walking down a slope, and that the reduction in stiffness is likely due to an increase in inhibitory force feedback. Our results further suggest that a body orientation signal derived from vestibular and neck proprioceptive information is responsible for the required muscular activation patterns as well as a reduction in limb stiffness. This increased compliance is consistent with the function of the distal limb to cushion the impact during the braking action of the antigravity musculature.
Figures

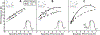
References
-
- Abelew TA, Miller MD, Cope TC, Nichols TR. 2000. Local loss of proprioception results in disruption of interjoint coordination during locomotion in the cat. J Neurophysiol 84:2709–2714. - PubMed
-
- Ahn AN, Full RJ. 2002. A motor and a brake: two leg extensor muscles acting at the same joint manage energy differently in a running insect. J Exp Biol 205:379–389. - PubMed
-
- Ashley MJ, Gryfe CI, Amies A. 1977. A longitudinal study of falls in an elderly population II. Some circumstances of falling. Age Ageing 6:211–220. - PubMed
-
- Bonasera SJ, Nichols TR. 1994. Mechanical actions of heterogenic reflexes linking long toe flexors with ankle and knee extensors of the cat hindlimb. J Neurophysiol 71:1096–1110. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

