PET imaging of bacterial infections with fluorine-18-labeled maltohexaose
- PMID: 25330976
- PMCID: PMC4430476
- DOI: 10.1002/anie.201408533
PET imaging of bacterial infections with fluorine-18-labeled maltohexaose
Abstract
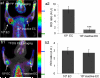
A positron emission tomography (PET) tracer composed of (18)F-labeled maltohexaose (MH(18)F) can image bacteria in vivo with a sensitivity and specificity that are orders of magnitude higher than those of fluorodeoxyglucose ((18)FDG). MH(18)F can detect early-stage infections composed of as few as 10(5) E. coli colony-forming units (CFUs), and can identify drug resistance in bacteria in vivo. MH(18)F has the potential to improve the diagnosis of bacterial infections given its unique combination of high specificity and sensitivity for bacteria.
Keywords: bacterial infections; maltodextrin transporters; maltohexaose; positron emission tomography; radiochemistry.
© 2014 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures






References
-
- Lipsky BA, Itani K, Norden C. G. Linezolid Diabetic Foot Infections Study. Clin. Infect. Dis. 2004;38:17–24. - PubMed
- Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, Dowell SF, File TM, Jr., Musher DM, Niederman MS, Torres A, Whitney CG. A. Infectious Diseases Society of, S. American Thoracic. Clin. Infect. Dis. 2007;44(Suppl 2):S27–72. - PMC - PubMed
-
- Glaudemans AW, Signore A. Eur. J. Nucl. Med. Mol. Imag. 2010;37:1986–1991. - PMC - PubMed
- Erba PA, Bandera F, Sollini M, Tascini C. J. Am. Coll. Cardiol. 2012;60:1435–1436. author reply 1437. - PubMed
- Keidar Z, Nitecki S. Semin. Nucl. Med. 2013;43:396–402. - PubMed
- del Rosal T, Goycochea WA, Mendez-Echevarria A, Garcia-Fernandez de Villalta M, Baquero-Artigao F, Coronado M, Marin MD, Albajara L. Eur. J. Pediatr. 2013;172:1111–1115. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

