Gelatin-chondroitin-6-sulfate-hyaluronic acid scaffold seeded with vascular endothelial growth factor 165 modified hair follicle stem cells as a three-dimensional skin substitute
- PMID: 25331352
- PMCID: PMC4535258
- DOI: 10.1186/scrt508
Gelatin-chondroitin-6-sulfate-hyaluronic acid scaffold seeded with vascular endothelial growth factor 165 modified hair follicle stem cells as a three-dimensional skin substitute
Abstract
Introduction: In the field of skin tissue engineering, gelatin-chondroitin-6-sulfate-hyaluronic acid (Gel-C6S-HA) stents are a suitable bio skin substitute. The purpose was to investigate the effect of genetically-modified hair follicle stem cells (HFSCs), combined with Gel-C6S-HA scaffolds, on the vascularization of tissue-engineered skin.
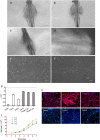
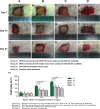
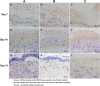
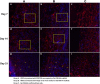
Methods: Three-dimensional (3D) Gel-C6S-HA scaffolds were prepared by freeze-drying. Vascular endothelial growth factor (VEGF) 165 gene-modified rat HFSCs (rHFSCs) were inoculated into the scaffolds and cultured for 7 days. Two bilateral full-thickness skin defects were created on the back of 18 Sprague-Dawley rats. Rats were randomly divided into four groups: Group A, HFSCs transduced with VEGF165 seeded onto Gel-C6S-HA scaffolds; Group B, HFSCs transduced with empty vector seeded onto Gel-C6S-HA scaffolds; Group C, Gel-C6S-HA scaffold only; Group D, Vaseline gauze dressing. These compositions were implanted onto the defects and harvested at 7, 14 and 21 days. Wound healing was assessed and compared among groups according to hematoxylin-eosin staining, CD31 expression, alpha smooth muscle actin (α-SMA) and major histocompatibility complex class I (MHC-I) immunohistochemistry, and microvessel density (MVD) count, to evaluate the new blood vessels.
Results: SEM revealed the Gel-C6S-HA scaffold was spongy and 3D, with an average pore diameter of 133.23 ± 43.36 μm. Cells seeded on scaffolds showed good adherent growth after 7 days culture. No significant difference in rHFSC morphology, adherence and proliferative capacity was found before and after transfection (P >0.05). After 14 and 21 days, the highest rate of wound healing was observed in Group A (P <0.05). Histological and immunological examination showed that after 21 days, MVD also reached a maximum in Group A (P <0.05). Therefore, the number of new blood vessels formed within the skin substitutes was greatest in Group A, followed by Group B. In Group C, only trace amounts of mature subcutaneous blood vessels were observed, and few subcutaneous tissue cells migrated into the scaffolds.
Conclusions: Tissue-engineered skin constructs, using 3D Gel-C6S-HA scaffolds seeded with VEGF165-modified rHFSCs, resulted in promotion of angiogenesis during wound healing and facilitation of vascularization in skin substitutes. This may be a novel approach for tissue-engineered skin substitutes.
Figures






Similar articles
-
Cross-linked collagen-chondroitin sulfate-hyaluronic acid imitating extracellular matrix as scaffold for dermal tissue engineering.Tissue Eng Part C Methods. 2010 Apr;16(2):269-79. doi: 10.1089/ten.TEC.2009.0161. Tissue Eng Part C Methods. 2010. PMID: 19530938
-
Silk fibroin/chondroitin sulfate/hyaluronic acid ternary scaffolds for dermal tissue reconstruction.Acta Biomater. 2013 Jun;9(6):6771-82. doi: 10.1016/j.actbio.2013.02.016. Epub 2013 Feb 16. Acta Biomater. 2013. PMID: 23419553
-
Feasibility of repairing skin defects by VEGF165 gene-modified iPS-HFSCs seeded on a 3D printed scaffold containing astragalus polysaccharide.J Cell Mol Med. 2023 Aug;27(15):2136-2149. doi: 10.1111/jcmm.17800. Epub 2023 Jun 1. J Cell Mol Med. 2023. PMID: 37264501 Free PMC article.
-
The Development of Hyaluronic Acids Used for Skin Tissue Regeneration.Curr Drug Deliv. 2021;18(7):836-846. doi: 10.2174/1567201817666201202094513. Curr Drug Deliv. 2021. PMID: 33267761 Review.
-
Hyaluronic Acid Role in Biomaterials Prevascularization.Adv Healthc Mater. 2024 Dec;13(30):e2402045. doi: 10.1002/adhm.202402045. Epub 2024 Sep 10. Adv Healthc Mater. 2024. PMID: 39254277 Review.
Cited by
-
Hybrid cellulose nanocrystal/alginate/gelatin scaffold with improved mechanical properties and guided wound healing.RSC Adv. 2019 Jul 25;9(40):22966-22979. doi: 10.1039/c9ra04026a. eCollection 2019 Jul 23. RSC Adv. 2019. PMID: 35548324 Free PMC article.
-
Hybrid Sponge-Like Scaffolds Based on Ulvan and Gelatin: Design, Characterization and Evaluation of Their Potential Use in Bone Tissue Engineering.Materials (Basel). 2020 Apr 9;13(7):1763. doi: 10.3390/ma13071763. Materials (Basel). 2020. PMID: 32283814 Free PMC article.
-
Biomaterials for Skin Substitutes.Adv Healthc Mater. 2018 Mar;7(5):10.1002/adhm.201700897. doi: 10.1002/adhm.201700897. Epub 2017 Dec 22. Adv Healthc Mater. 2018. PMID: 29271580 Free PMC article. Review.
-
In Vitro and In Vivo Evaluation of Carboxymethyl Cellulose Scaffolds for Bone Tissue Engineering Applications.ACS Omega. 2021 Jan 4;6(2):1246-1253. doi: 10.1021/acsomega.0c04551. eCollection 2021 Jan 19. ACS Omega. 2021. PMID: 33490783 Free PMC article.
-
Gelatin-polysaccharide composite scaffolds for 3D cell culture and tissue engineering: Towards natural therapeutics.Bioeng Transl Med. 2018 Dec 28;4(1):96-115. doi: 10.1002/btm2.10124. eCollection 2019 Jan. Bioeng Transl Med. 2018. PMID: 30680322 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

