Kinetic evidence against partitioning of the ubiquinone pool and the catalytic relevance of respiratory-chain supercomplexes
- PMID: 25331896
- PMCID: PMC4226120
- DOI: 10.1073/pnas.1413855111
Kinetic evidence against partitioning of the ubiquinone pool and the catalytic relevance of respiratory-chain supercomplexes
Abstract
In mitochondria, four respiratory-chain complexes drive oxidative phosphorylation by sustaining a proton-motive force across the inner membrane that is used to synthesize ATP. The question of how the densely packed proteins of the inner membrane are organized to optimize structure and function has returned to prominence with the characterization of respiratory-chain supercomplexes. Supercomplexes are increasingly accepted structural entities, but their functional and catalytic advantages are disputed. Notably, substrate "channeling" between the enzymes in supercomplexes has been proposed to confer a kinetic advantage, relative to the rate provided by a freely accessible, common substrate pool. Here, we focus on the mitochondrial ubiquinone/ubiquinol pool. We formulate and test three conceptually simple predictions of the behavior of the mammalian respiratory chain that depend on whether channeling in supercomplexes is kinetically important, and on whether the ubiquinone pool is partitioned between pathways. Our spectroscopic and kinetic experiments demonstrate how the metabolic pathways for NADH and succinate oxidation communicate and catalyze via a single, universally accessible ubiquinone/ubiquinol pool that is not partitioned or channeled. We reevaluate the major piece of contrary evidence from flux control analysis and find that the conclusion of substrate channeling arises from the particular behavior of a single inhibitor; we explain why different inhibitors behave differently and show that a robust flux control analysis provides no evidence for channeling. Finally, we discuss how the formation of respiratory-chain supercomplexes may confer alternative advantages on energy-converting membranes.
Keywords: mitochondria; respirasome; respiratory chain; supercomplex; ubiquinone.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

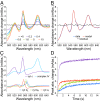
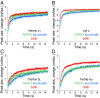

References
-
- Kröger A, Klingenberg M. The kinetics of the redox reactions of ubiquinone related to the electron-transport activity in the respiratory chain. Eur J Biochem. 1973;34(2):358–368. - PubMed
-
- Chazotte B, Hackenbrock CR. The multicollisional, obstructed, long-range diffusional nature of mitochondrial electron transport. J Biol Chem. 1988;263(28):14359–14367. - PubMed
-
- Acín-Pérez R, Fernández-Silva P, Peleato ML, Pérez-Martos A, Enriquez JA. Respiratory active mitochondrial supercomplexes. Mol Cell. 2008;32(4):529–539. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

