Potential antigenic explanation for atypical H1N1 infections among middle-aged adults during the 2013-2014 influenza season
- PMID: 25331901
- PMCID: PMC4226110
- DOI: 10.1073/pnas.1409171111
Potential antigenic explanation for atypical H1N1 infections among middle-aged adults during the 2013-2014 influenza season
Abstract
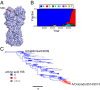
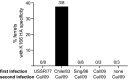
Influenza viruses typically cause the most severe disease in children and elderly individuals. However, H1N1 viruses disproportionately affected middle-aged adults during the 2013-2014 influenza season. Although H1N1 viruses recently acquired several mutations in the hemagglutinin (HA) glycoprotein, classic serological tests used by surveillance laboratories indicate that these mutations do not change antigenic properties of the virus. Here, we show that one of these mutations is located in a region of HA targeted by antibodies elicited in many middle-aged adults. We find that over 42% of individuals born between 1965 and 1979 possess antibodies that recognize this region of HA. Our findings offer a possible antigenic explanation of why middle-aged adults were highly susceptible to H1N1 viruses during the 2013-2014 influenza season. Our data further suggest that a drifted H1N1 strain should be included in future influenza vaccines to potentially reduce morbidity and mortality in this age group.
Keywords: antibody; antigenic drift; hemagglutinin; influenza; vaccine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Dawood FS, et al. Novel Swine-Origin Influenza A (H1N1) Virus Investigation Team Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009;360(25):2605–2615. - PubMed
-
- Smith GJ, et al. Origins and evolutionary genomics of the 2009 swine-origin H1N1 influenza A epidemic. Nature. 2009;459(7250):1122–1125. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HSN266200700006C/PHS HHS/United States
- U19 AI090023/AI/NIAID NIH HHS/United States
- 1P01AI097092-01/AI/NIAID NIH HHS/United States
- P51 OD011132/OD/NIH HHS/United States
- R01GM102198/GM/NIGMS NIH HHS/United States
- T32 AI074492/AI/NIAID NIH HHS/United States
- R01 AI113047/AI/NIAID NIH HHS/United States
- R01 GM102198/GM/NIGMS NIH HHS/United States
- 1R01AI113047/AI/NIAID NIH HHS/United States
- P30CA010815/CA/NCI NIH HHS/United States
- P30 CA010815/CA/NCI NIH HHS/United States
- R01 AI108686/AI/NIAID NIH HHS/United States
- T32 CA009171/CA/NCI NIH HHS/United States
- P01 AI097092/AI/NIAID NIH HHS/United States
- U19 AI082724/AI/NIAID NIH HHS/United States
- 1R01AI108686/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

