mTOR activation promotes plasma cell differentiation and bypasses XBP-1 for immunoglobulin secretion
- PMID: 25332234
- PMCID: PMC4295374
- DOI: 10.1128/MCB.01187-14
mTOR activation promotes plasma cell differentiation and bypasses XBP-1 for immunoglobulin secretion
Abstract
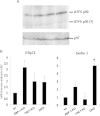
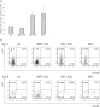
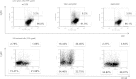
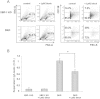
Plasma cells (PCs) are responsible for the secretion of antibodies. The development of fully functional PCs relies on the activation of the inositol-requiring enzyme 1/X-box binding protein 1 (IRE1/XBP-1) arm of the unfolded protein response (UPR). XBP-1-deficient PCs secrete antibodies poorly and exhibit distensions of the endoplasmic reticulum (ER). The kinase mammalian target of rapamycin (mTOR) promotes anabolic activities and is negatively regulated by the tuberous sclerosis complex (TSC). Deletion of TSC1 renders mTOR hyperactive. To explore the relationship between mTOR and the UPR in PC development and function, mice with conditional deletions of XBP-1 and/or TSC1 in their B cell lineage were generated. Deletion of TSC1 enhanced Ig synthesis and promoted differentiation into PCs independently of XBP-1, as evidenced by comparison of TSC1/XBP-1 double-knockout (DKO) PCs to XBP-1 knockout (KO) PCs. The typical morphological abnormalities of the ER in XBP-1 KO PCs were alleviated in the DKO PCs. Expression profiling identified the glycoprotein Ly6C as an mTOR target. Ly6C expression contributed to the enhanced Ig secretion from DKO PCs. Our data reveal a functional overlap between mTOR and the UPR in promoting PC development. In addition to the classical mTOR role in promoting protein synthesis, the mechanism entails transcription regulation of accessory molecules, such as Ly6C.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
