The mitochondrial complex V-associated large-conductance inner membrane current is regulated by cyclosporine and dexpramipexole
- PMID: 25332381
- PMCID: PMC4279080
- DOI: 10.1124/mol.114.095661
The mitochondrial complex V-associated large-conductance inner membrane current is regulated by cyclosporine and dexpramipexole
Abstract
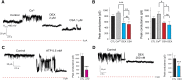
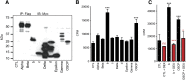
Inefficiency of oxidative phosphorylation can result from futile leak conductance through the inner mitochondrial membrane. Stress or injury may exacerbate this leak conductance, putting cells, and particularly neurons, at risk of dysfunction and even death when energy demand exceeds cellular energy production. Using a novel method, we have recently described an ion conductance consistent with mitochondrial permeability transition pore (mPTP) within the c-subunit of the ATP synthase. Excitotoxicity, reactive oxygen species-producing stimuli, or elevated mitochondrial matrix calcium opens the channel, which is inhibited by cyclosporine A and ATP/ADP. Here we show that ATP and the neuroprotective drug dexpramipexole (DEX) inhibited an ion conductance consistent with this c-subunit channel (mPTP) in brain-derived submitochondrial vesicles (SMVs) enriched for F1FO ATP synthase (complex V). Treatment of SMVs with urea denatured extramembrane components of complex V, eliminated DEX- but not ATP-mediated current inhibition, and reduced binding of [(14)C]DEX. Direct effects of DEX on the synthesis and hydrolysis of ATP by complex V suggest that interaction of the compound with its target results in functional conformational changes in the enzyme complex. [(14)C]DEX bound specifically to purified recombinant b and oligomycin sensitivity-conferring protein subunits of the mitochondrial F1FO ATP synthase. Previous data indicate that DEX increased the efficiency of energy production in cells, including neurons. Taken together, these studies suggest that modulation of a complex V-associated inner mitochondrial membrane current is metabolically important and may represent an avenue for the development of new therapeutics for neurodegenerative disorders.
Copyright © 2014 by The American Society for Pharmacology and Experimental Therapeutics.
Figures



References
-
- Alavian KN, Beutner G, Lazrove E, Sacchetti S, Park HA, Licznerski P, Li H, Nabili P, Hockensmith K, Graham M, et al. (2014) An uncoupling channel within the c-subunit ring of the F1FO ATP synthase is the mitochondrial permeability transition pore. Proc Natl Acad Sci USA 111:10580–10585. - PMC - PubMed
-
- Albers DS, Beal MF. (2000) Mitochondrial dysfunction and oxidative stress in aging and neurodegenerative disease. J Neural Transm Suppl 59:133–154. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

