Updates in Rhea--a manually curated resource of biochemical reactions
- PMID: 25332395
- PMCID: PMC4384025
- DOI: 10.1093/nar/gku961
Updates in Rhea--a manually curated resource of biochemical reactions
Abstract
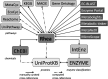
Rhea (http://www.ebi.ac.uk/rhea) is a comprehensive and non-redundant resource of expert-curated biochemical reactions described using species from the ChEBI (Chemical Entities of Biological Interest) ontology of small molecules. Rhea has been designed for the functional annotation of enzymes and the description of genome-scale metabolic networks, providing stoichiometrically balanced enzyme-catalyzed reactions (covering the IUBMB Enzyme Nomenclature list and additional reactions), transport reactions and spontaneously occurring reactions. Rhea reactions are extensively curated with links to source literature and are mapped to other publicly available enzyme and pathway databases such as Reactome, BioCyc, KEGG and UniPathway, through manual curation and computational methods. Here we describe developments in Rhea since our last report in the 2012 database issue of Nucleic Acids Research. These include significant growth in the number of Rhea reactions and the inclusion of reactions involving complex macromolecules such as proteins, nucleic acids and other polymers that lie outside the scope of ChEBI. Together these developments will significantly increase the utility of Rhea as a tool for the description, analysis and reconciliation of genome-scale metabolic models.
© The Author(s) 2014. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures


References
-
- Belda E., Sekowska A., Le Fevre F., Morgat A., Mornico D., Ouzounis C., Vallenet D., Medigue C., Danchin A. An updated metabolic view of the Bacillus subtilis 168 genome. Microbiology. 2013;159:757–770. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

