SIRT3 and SIRT4 are mitochondrial tumor suppressor proteins that connect mitochondrial metabolism and carcinogenesis
- PMID: 25332769
- PMCID: PMC4203689
- DOI: 10.1186/2049-3002-2-15
SIRT3 and SIRT4 are mitochondrial tumor suppressor proteins that connect mitochondrial metabolism and carcinogenesis
Abstract
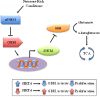
It is a well-established scientific observation that mammalian cells contain fidelity proteins that appear to protect against and adapt to various forms of endogenous and exogenous cellular conditions. Loss of function or genetic mutation of these fidelity proteins has also been shown to create a cellular environment that is permissive for the development of tumors, suggesting that these proteins also function as tumor suppressors (TSs). While the first identified TSs were confined to either the nucleus and/or the cytoplasm, it seemed logical to hypothesize that the mitochondria may also contain fidelity proteins that serve as TSs. In this regard, it now appears clear that at least two mitochondrial sirtuins function as sensing, watchdog, or TS proteins in vitro, in vivo, and in human tumor samples. In addition, these new results demonstrate that the mitochondrial anti-aging or fidelity/sensing proteins, SIRT3 and SIRT4, respond to changes in cellular nutrient status to alter the enzymatic activity of specific downstream targets to maintain energy production that matches energy availability and ATP consumption. As such, it is proposed that loss of function or genetic deletion of these mitochondrial genes results in a mismatch of mitochondrial energy metabolism, culminating in a cell phenotype permissive for transformation and tumorigenesis. In addition, these findings clearly suggest that loss of proper mitochondrial metabolism, via loss of SIRT3 and SIRT4, is sufficient to promote carcinogenesis.
Keywords: Acetylation; Acetylome; Carcinogenesis; SIRT3; SIRT4.
Figures


References
-
- Uren AG, Kool J, Matentzoglu K, de Ridder J, Mattison J, van Uitert M, Lagcher W, Sie D, Tanger E, Cox T, Reinders M, Hubbard TJ, Rogers J, Jonkers J, Wessels L, Adams DJ, van Lohuizen M, Berns A. Large-scale mutagenesis in p19(ARF)- and p53-deficient mice identifies cancer genes and their collaborative networks. Cell. 2008;133(4):727–741. doi: 10.1016/j.cell.2008.03.021. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

