Percutaneous left atrial appendage occlusion for stroke prevention in atrial fibrillation: an update
- PMID: 25332785
- PMCID: PMC4195925
- DOI: 10.1136/openhrt-2013-000020
Percutaneous left atrial appendage occlusion for stroke prevention in atrial fibrillation: an update
Abstract
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia encountered in clinical practice. One of its most devastating complications is the development of thromboembolism leading to fatal or disabling stroke. Oral anticoagulation (OAC, warfarin) is the standard treatment for stroke prevention in patients with AF with an increased stroke risk. However, there are several obstacles to long-term OAC therapy, including the risk of serious bleeding, several drug-drug interactions and the need for frequent blood testing. Although newer oral anticoagulants have been developed, these drugs also face issues of major bleeding and non-compliance. Therefore, alternative treatment options for stroke prevention in patients with AF with a high stroke risk are needed. Percutaneous left atrial appendage (LAA) occlusion is an evolving therapy, which should be taken into consideration in those patients with non-valvular AF with a high stroke risk and contraindications for OAC. This article aims to discuss the rationale for LAA closure, the available LAA occlusion devices and their clinical evidence until now. Moreover, we discuss the importance of proper patient selection, the role of various imaging techniques and the need for a more tailored postprocedural antithrombotic therapy.
Keywords: Allied Specialities.
Figures


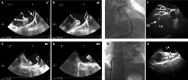
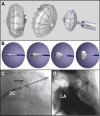
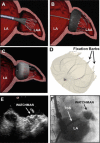
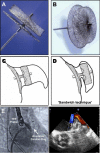
References
-
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 1991;22:983–8 - PubMed
-
- Furberg CD, Psaty BM, Manolio TA, et al. Prevalence of atrial fibrillation in elderly subjects—the Cardiovascular Health Study. Am J Cardiol 1994;74:236–41 - PubMed
-
- Go AS, Hylek EM, Philips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001;285:2370–5 - PubMed
-
- Feinberg WM, Blackshear JL, Laupacis , et al. Prevalence, age distribution, and gender of patients with atrial fibrillation. Analysis and implications. Arch Intern Med 1995;155:469–73 - PubMed
-
- (No Author used). Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Analysis of pooled data from five randomized controlled trials. Arch Intern Med 1994;154:1449–57 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
