First-generation versus second-generation drug-eluting stents in current clinical practice: updated evidence from a comprehensive meta-analysis of randomised clinical trials comprising 31 379 patients
- PMID: 25332803
- PMCID: PMC4189321
- DOI: 10.1136/openhrt-2014-000064
First-generation versus second-generation drug-eluting stents in current clinical practice: updated evidence from a comprehensive meta-analysis of randomised clinical trials comprising 31 379 patients
Abstract
Background: First-generation drug-eluting stents (DES) have become the most widely used devices worldwide for management of coronary artery disease. As remote follow-up data were becoming available, concerns emerged in regard to their long-term safety. Second-generation DES were designed to overcome safety issues, but the results of randomised clinical trials remain conflicting.
Methods: We compared the safety and efficacy of first-generation versus second-generation Food and Drug Administration approved DES; the following devices were included: first-generation sirolimus-eluting stent (SES) and paclitaxel-eluting stents (PES); second-generation everolimus-eluting stent (EES), zotarolimus-eluting stent Endeavor and ZES-Resolute (ZES-R). Prespecified safety end points comprised ≤1 and >1 year: overall and cardiac mortality, myocardial infarction (MI), definite/definite or probable ST; efficacy end points were target lesion revascularisation and target vessel revascularisation. Composite end points were analysed as well.
Results: 33 randomised controlled trials involving 31 379 patients with stable coronary artery disease or acute coronary syndrome undergoing DES implantation were retrieved. No differences in mortality among devices were found. In the overall class comparison, second-generation DES were associated with a 22% reduction of odds of MI at short-term OR 0.77 (95% CI 0.68 to 0.89) p=0.0002; EES reduced the odds of definite-probable ST compared with PES: OR 0.33 (95% CI 0.15 to 0.73) p=0.006; First-generation SES along with second-generation EES and ZES-R showed similar efficacy in decreasing the odds of repeat revascularisation.
Conclusions: Second-generation EES and ZES-R offer similar levels of efficacy compared with first-generation SES, but are more effective than PES; however, only second-generation EES significantly reduced the incidence of MI and ST, and therefore should be perceived as the safest DES to date.
Keywords: CORONARY ARTERY DISEASE; EBM; INTERVENTIONAL CARDIOLOGY.
Figures

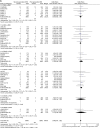

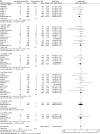





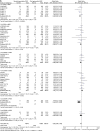

References
-
- Greenhalgh J, Hockenhull J, Rao N, et al. Drug-eluting stents versus bare metal stents for angina or acute coronary syndromes. Cochrane Database Syst Rev 2010;(5):CD004587. - PubMed
-
- Camenzind E, Steg PG, Wijns W. Stent thrombosis late after implantation of first-generation drug-eluting stents: a cause for concern. Circulation 2007;115:1440–55; discussion 1455 - PubMed
-
- Daemen J, Wenaweser P, Tsuchida K, et al. Early and late coronary stent thrombosis of sirolimus-eluting and paclitaxel-eluting stents in routine clinical practice: data from a large two-institutional cohort study. Lancet 2007;369:667–78 - PubMed
-
- Joner M, Finn AV, Farb A, et al. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol 2006;48:193–202 - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
