Choosing inclusion criteria that minimize the time and cost of clinical trials
- PMID: 25332910
- PMCID: PMC4202479
- DOI: 10.5662/wjm.v4.i2.109
Choosing inclusion criteria that minimize the time and cost of clinical trials
Abstract
Aim: To present statistical tools to model and optimize the cost of a randomized clinical trial as a function of the stringency of patient inclusion criteria.
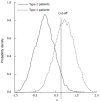
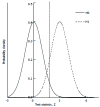
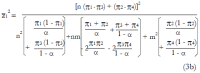
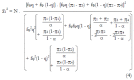
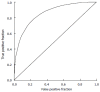
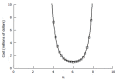
Methods: We consider a two treatment, dichotomous outcome trial that includes a proportion of patients who are strong responders to the tested intervention. Patients are screened for inclusion using an arbitrary number of test results that are combined into an aggregate suitability score. The screening score is regarded as a diagnostic test for the responsive phenotype, having a specific cutoff value for inclusion and a particular sensitivity and specificity. The cutoff is a measure of stringency of inclusion criteria. Total cost is modeled as a function of the cutoff value, number of patients screened, the number of patients included, the case occurrence rate, response probabilities for control and experimental treatments, and the trial duration required to produce a statistically significant result with a specified power. Regression methods are developed to estimate relevant model parameters from pilot data in an adaptive trial design.
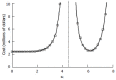
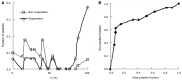
Results: The patient numbers and total cost are strongly related to the choice of the cutoff for inclusion. Clear cost minimums exist between 5.6 and 6.1 on a representative 10-point scale of exclusiveness. Potential cost savings for typical trial scenarios range in millions of dollars. As the response rate for controls approaches 50%, the proper choice of inclusion criteria can mean the difference between a successful trial and a failed trial.
Conclusion: Early formal estimation of optimal inclusion criteria allows planning of clinical trials to avoid high costs, excessive delays, and moral hazards of Type II errors.
Keywords: Adaptive trial designs; Biomarkers; Clinical trials; Device; Drug therapy; Ethics; Methodology; Optimal allocation; Personalized medicine; Sequential design.
Figures
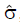
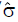

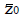
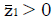
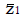
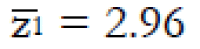
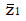

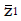
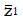
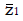


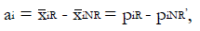
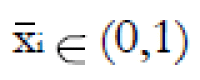
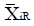
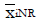
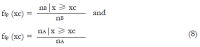
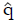
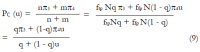
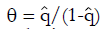
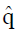


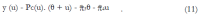
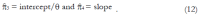
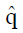

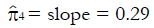
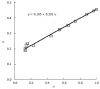
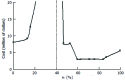
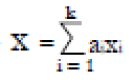
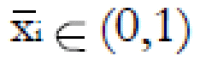
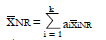
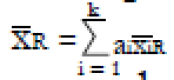
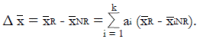
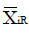
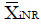
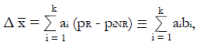
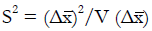
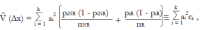
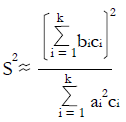
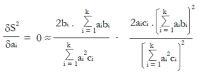
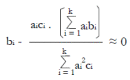
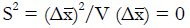
References
-
- Anderson JE, Hansen LL, Mooren FC, Post M, Hug H, Zuse A, Los M. Methods and biomarkers for the diagnosis and prognosis of cancer and other diseases: towards personalized medicine. Drug Resist Updat. 2006;9:198–210. - PubMed
-
- Ross JS, Slodkowska EA, Symmans WF, Pusztai L, Ravdin PM, Hortobagyi GN. The HER-2 receptor and breast cancer: ten years of targeted anti-HER-2 therapy and personalized medicine. Oncologist. 2009;14:320–368. - PubMed
-
- van’t Veer LJ, Bernards R. Enabling personalized cancer medicine through analysis of gene-expression patterns. Nature. 2008;452:564–570. - PubMed
-
- Yao Q, Wei LJ. Play the winner for phase II/III clinical trials. Stat Med. 1996;15:2413–223; discussion 2413-223;. - PubMed
-
- Bauer P, Köhne K. Evaluation of experiments with adaptive interim analyses. Biometrics. 1994;50:1029–1041. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

