Systematic evaluation of the patient-reported outcome (PRO) content of clinical trial protocols
- PMID: 25333349
- PMCID: PMC4198237
- DOI: 10.1371/journal.pone.0110229
Systematic evaluation of the patient-reported outcome (PRO) content of clinical trial protocols
Abstract
Background: Qualitative evidence suggests patient-reported outcome (PRO) information is frequently absent from clinical trial protocols, potentially leading to inconsistent PRO data collection and risking bias. Direct evidence regarding PRO trial protocol content is lacking. The aim of this study was to systematically evaluate the PRO-specific content of UK National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme trial protocols.
Methods and findings: We conducted an electronic search of the NIHR HTA programme database (inception to August 2013) for protocols describing a randomised controlled trial including a primary/secondary PRO. Two investigators independently reviewed the content of each protocol, using a specially constructed PRO-specific protocol checklist, alongside the 'Standard Protocol Items: Recommendations for Interventional Trials' (SPIRIT) checklist. Disagreements were resolved through discussion with a third investigator. 75 trial protocols were included in the analysis. Protocols included a mean of 32/51 (63%) SPIRIT recommendations (range 16-41, SD 5.62) and 11/33 (33%) PRO-specific items (range 4-18, SD 3.56). Over half (61%) of the PRO items were incomplete. Protocols containing a primary PRO included slightly more PRO checklist items (mean 14/33 (43%)). PRO protocol content was not associated with general protocol completeness; thus, protocols judged as relatively 'complete' using SPIRIT were still likely to have omitted a large proportion of PRO checklist items.
Conclusions: The PRO components of HTA clinical trial protocols require improvement. Information on the PRO rationale/hypothesis, data collection methods, training and management was often absent. This low compliance is unsurprising; evidence shows existing PRO guidance for protocol developers remains difficult to access and lacks consistency. Study findings suggest there are a number of PRO protocol checklist items that are not fully addressed by the current SPIRIT statement. We therefore advocate the development of consensus-based supplementary guidelines, aimed at improving the completeness and quality of PRO content in clinical trial protocols.
Conflict of interest statement
Figures
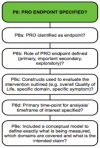
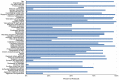

References
-
- Ahmed S, Berzon RA, Revicki DA, Lenderking WR, Moinpour CM, et al. (2012) The Use of Patient-reported Outcomes (PRO) Within Comparative Effectiveness Research: Implications for Clinical Practice and Health Care Policy. Medical Care 50: 1060–1070. - PubMed
-
- Health Do (2010) Equity and excellence: Liberating the NHS. In: Health Do, editor.
-
- Calvert MJ, Freemantle N (2003) Use of health-related quality of life in prescribing research. Part 1: why evaluate health-related quality of life? Journal of Clinical Pharmacy and Therapeutics 28: 513–521. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

