Lifespan based pharmacokinetic-pharmacodynamic model of tumor growth inhibition by anticancer therapeutics
- PMID: 25333487
- PMCID: PMC4204849
- DOI: 10.1371/journal.pone.0109747
Lifespan based pharmacokinetic-pharmacodynamic model of tumor growth inhibition by anticancer therapeutics
Abstract
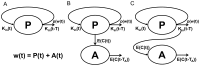
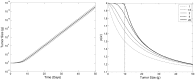
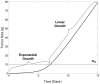
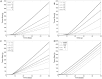
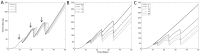
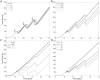
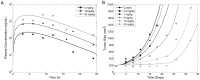
Accurate prediction of tumor growth is critical in modeling the effects of anti-tumor agents. Popular models of tumor growth inhibition (TGI) generally offer empirical description of tumor growth. We propose a lifespan-based tumor growth inhibition (LS TGI) model that describes tumor growth in a xenograft mouse model, on the basis of cellular lifespan T. At the end of the lifespan, cells divide, and to account for tumor burden on growth, we introduce a cell division efficiency function that is negatively affected by tumor size. The LS TGI model capability to describe dynamic growth characteristics is similar to many empirical TGI models. Our model describes anti-cancer drug effect as a dose-dependent shift of proliferating tumor cells into a non-proliferating population that die after an altered lifespan TA. Sensitivity analysis indicated that all model parameters are identifiable. The model was validated through case studies of xenograft mouse tumor growth. Data from paclitaxel mediated tumor inhibition was well described by the LS TGI model, and model parameters were estimated with high precision. A study involving a protein casein kinase 2 inhibitor, AZ968, contained tumor growth data that only exhibited linear growth kinetics. The LS TGI model accurately described the linear growth data and estimated the potency of AZ968 that was very similar to the estimate from an established TGI model. In the case study of AZD1208, a pan-Pim inhibitor, the doubling time was not estimable from the control data. By fixing the parameter to the reported in vitro value of the tumor cell doubling time, the model was still able to fit the data well and estimated the remaining parameters with high precision. We have developed a mechanistic model that describes tumor growth based on cell division and has the flexibility to describe tumor data with diverse growth kinetics.
Conflict of interest statement
Figures


References
-
- Danhof M, de Lange ECM, Della Pasqua OE, Ploeger BA, Voskuyl RA (2008) Mechanism-based pharmacokinetic-pharmacodynamic (PK-PD) modeling in translational drug research. Trends in Pharmacological Sciences 29: 186–191. - PubMed
-
- Schabel FM (1969) The Use of Tumor Growth Kinetics in Planning “Curative” Chemotherapy of Advanced Solid Tumors. Cancer Res 29: 2384–2389. - PubMed
-
- Bajzer Z, Marušić M, Vuk-Pavlović S (1996) Conceptual frameworks for mathematical modeling of tumor growth dynamics. Mathematical and Computer Modelling 23: 31–46.
-
- Sachs RK, Hlatky LR, Hahnfeldt P (2001) Simple ODE models of tumor growth and anti-angiogenic or radiation treatment. Mathematical and Computer Modelling 33: 1297–1305.
-
- Frei E 3rd, Bickers JN, Hewlett JS, Lane M, Leary WV, et al. (1969) Dose schedule and antitumor studies of arabinosyl cytosine (NSC 63878). Cancer Res 29: 1325–1332. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

