A role for site-specific phosphorylation of mouse progesterone receptor at serine 191 in vivo
- PMID: 25333515
- PMCID: PMC4250360
- DOI: 10.1210/me.2014-1206
A role for site-specific phosphorylation of mouse progesterone receptor at serine 191 in vivo
Abstract
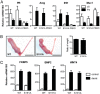
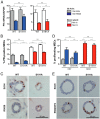
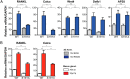
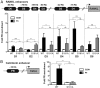
Progesterone receptors (PRs) are phosphorylated on multiple sites, and a variety of roles for phosphorylation have been suggested by cell-based studies. Previous studies using PR-null mice have shown that PR plays an important role in female fertility, regulation of uterine growth, the uterine decidualization response, and proliferation as well as ductal side-branching and alveologenesis in the mammary gland. To study the role of PR phosphorylation in vivo, a mouse was engineered with homozygous replacement of PR with a PR serine-to-alanine mutation at amino acid 191. No overt phenotypes were observed in the mammary glands or uteri of PR S191A treated with progesterone (P4). In contrast, although PR S191A mice were fertile, litters were 19% smaller than wild type and the estrous cycle was lengthened slightly. Moreover, P4-dependent gene regulation in primary mammary epithelial cells (MECs) was altered in a gene-selective manner. MECs derived from wild type and PR S191A mice were grown in a three-dimensional culture. Both formed acinar structures that were morphologically similar, and proliferation was stimulated equally by P4. However, P4 induction of receptor activator of nuclear factor-κB ligand and calcitonin was selectively reduced in S191A cultures. These differences were confirmed in freshly isolated MECs. Chromatin immunoprecipitation analysis showed that the binding of S191A PR to some of the receptor activator of nuclear factor-κB ligand enhancers and a calcitonin enhancer was substantially reduced. Thus, the elimination of a single phosphorylation site is sufficient to modulate PR activity in vivo. PR contains many phosphorylation sites, and the coordinate regulation of multiple sites is a potential mechanism for selective modulation of PR function.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

