GnRH evokes localized subplasmalemmal calcium signaling in gonadotropes
- PMID: 25333516
- PMCID: PMC4250365
- DOI: 10.1210/me.2014-1208
GnRH evokes localized subplasmalemmal calcium signaling in gonadotropes
Abstract
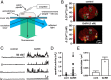
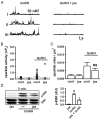
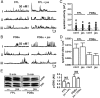
The binding of GnRH to its receptor initiates signaling cascades in gonadotropes, which result in enhanced LH and FSH biosynthesis and secretion. This process is necessary for follicular maturation and ovulation. Calcium influx activates MAPKs, which lead to increased transcription of LH and FSH genes. Previous research suggests that two MAPK signaling pathways, ERK and jun-N-terminal kinase, are activated by either calcium influx through L-type calcium channels or by global calcium signals originating from intracellular stores, respectively. Here we continued this investigation to further elucidate molecular mechanisms transducing GnRH receptor stimulation to ERK activation. Although it is known that GnRH activation of ERK requires calcium influx through L-type calcium channels, direct evidence supporting an underlying local calcium signaling mechanism was lacking. Here we used a combination of electrophysiology and total internal reflection fluorescence microscopy to visualize discrete sites of calcium influx (calcium sparklets) in gonadotrope-derived αT3-1 cells in real time. GnRH increased localized calcium influx and promoted ERK activation. The L-type calcium channel agonist FPL 64176 enhanced calcium sparklets and ERK activation in a manner indistinguishable from GnRH. Conversely, the L-type calcium channel antagonist nicardipine inhibited not only localized calcium sparklets but also ERK activation in response to GnRH. GnRH-dependent stimulation of L-type calcium channels was found to require protein kinase C and a dynamic actin cytoskeleton. Taken together, we provide the first direct evidence for localized L-type calcium channel signaling in αT3-1 cells and demonstrate the utility of our approach for investigating signaling mechanisms and cellular organization in gonadotropes.
Figures


References
-
- Counis R, Laverrière JN, Garrel G, et al. Gonadotropin-releasing hormone and the control of gonadotrope function. Reprod Nutr Dev. 2005;45(3):243–254. - PubMed
-
- Grosse R, Schmid A, Schoneberg T, et al. Gonadotropin-releasing hormone receptor initiates multiple signaling pathways by exclusively coupling to G(q/11) proteins. J Biol Chem. 2000;275(13):9193–9200. - PubMed
-
- Naor Z. Signaling by G-protein-coupled receptor (GPCR): studies on the GnRH receptor. Front Neuroendocrinol. 2009;30(1):10–29. - PubMed
-
- Mulvaney JM, Zhang T, Fewtrell C, Roberson MS. Calcium influx through L-type channels is required for selective activation of extracellular signal-regulated kinase by gonadotropin-releasing hormone. J Biol Chem. 1999;274(42):29796–29804. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

