A synaptic mechanism for temporal filtering of visual signals
- PMID: 25333637
- PMCID: PMC4205119
- DOI: 10.1371/journal.pbio.1001972
A synaptic mechanism for temporal filtering of visual signals
Abstract
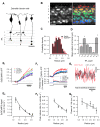
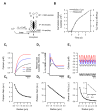
The visual system transmits information about fast and slow changes in light intensity through separate neural pathways. We used in vivo imaging to investigate how bipolar cells transmit these signals to the inner retina. We found that the volume of the synaptic terminal is an intrinsic property that contributes to different temporal filters. Individual cells transmit through multiple terminals varying in size, but smaller terminals generate faster and larger calcium transients to trigger vesicle release with higher initial gain, followed by more profound adaptation. Smaller terminals transmitted higher stimulus frequencies more effectively. Modeling global calcium dynamics triggering vesicle release indicated that variations in the volume of presynaptic compartments contribute directly to all these differences in response dynamics. These results indicate how one neuron can transmit different temporal components in the visual signal through synaptic terminals of varying geometries with different adaptational properties.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





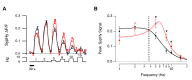
 For large terminals, A = 0.22±0.02, fo = 4.96±0.45, Q = 0.78±0.17. For small terminals A = 0.16±0.02, fo = 6.43±0.27, Q = 1.57±0.37. Asterisks mark significantly different responses at a given frequency, as evaluated by Mann-Whitney U test (p<0.05).
For large terminals, A = 0.22±0.02, fo = 4.96±0.45, Q = 0.78±0.17. For small terminals A = 0.16±0.02, fo = 6.43±0.27, Q = 1.57±0.37. Asterisks mark significantly different responses at a given frequency, as evaluated by Mann-Whitney U test (p<0.05).References
-
- Masland RH (2001) The fundamental plan of the retina. Nat Neurosci 4: 877–886. - PubMed
-
- Roska B, Werblin F (2001) Vertical interactions across ten parallel, stacked representations in the mammalian retina. Nature 410: 583–587. - PubMed
-
- Wässle H (2004) Parallel processing in the mammalian retina. Nat Rev Neurosci 5: 747–757. - PubMed
-
- Euler T, Haverkamp S, Schubert T, Baden T (2014) Retinal bipolar cells: elementary building blocks of vision. Nat Rev Neurosci 15: 507–519. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

