A solvent-free thermosponge nanoparticle platform for efficient delivery of labile proteins
- PMID: 25333768
- PMCID: PMC4245989
- DOI: 10.1021/nl502994y
A solvent-free thermosponge nanoparticle platform for efficient delivery of labile proteins
Abstract
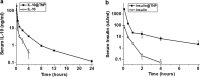
Protein therapeutics have gained attention recently for treatment of a myriad of human diseases due to their high potency and unique mechanisms of action. We present the development of a novel polymeric thermosponge nanoparticle for efficient delivery of labile proteins using a solvent-free polymer thermo-expansion mechanism with clinical potential, capable of effectively delivering a range of therapeutic proteins in a sustained manner with no loss of bioactivity, with improved biological half-lives and efficacy in vivo.
Keywords: Nanoparticles; biologics; solvent-free; therapeutic proteins; thermosponge.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

